当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rh-catalyzed asymmetric allylation of tert-butanesulfinamide
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-16 , DOI: 10.1039/d4qo00986j Min Liu 1 , Changkun Li 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-16 , DOI: 10.1039/d4qo00986j Min Liu 1 , Changkun Li 1
Affiliation
|
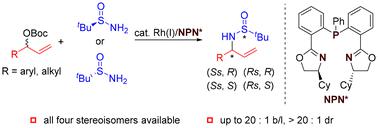
Chiral allylic tert-butanesulfinamides were developed as bidentate sulfur–olefin ligands for rhodium. The synthesis of such ligands is normally based on diastereoselective addition or reduction. The preparation of the diastereomer of allylic tert-butanesulfinamides is underdeveloped and the influence of the second chiral center is not fully examined. We report the Rh-catalyzed asymmetric allylation of tert-butanesulfinamides to synthesize all four stereoisomers of allylic tert-butanesulfinamides. The application of the cyclohexyl-substituted bisoxazolinephosphine ligand is the key to control the regioselectivity.
中文翻译:

Rh 催化叔丁亚磺酰胺的不对称烯丙基化
手性烯丙基叔丁亚磺酰胺被开发为铑的二齿硫-烯烃配体。此类配体的合成通常基于非对映选择性加成或还原。烯丙基叔丁烷亚磺酰胺非对映异构体的制备尚不成熟,第二手性中心的影响尚未得到充分研究。我们报道了 Rh 催化的叔丁烷亚磺酰胺的不对称烯丙基化反应,合成了烯丙基叔丁亚磺酰胺的所有四种立体异构体。环己基取代的双恶唑啉膦配体的应用是控制区域选择性的关键。
更新日期:2024-07-16
中文翻译:

Rh 催化叔丁亚磺酰胺的不对称烯丙基化
手性烯丙基叔丁亚磺酰胺被开发为铑的二齿硫-烯烃配体。此类配体的合成通常基于非对映选择性加成或还原。烯丙基叔丁烷亚磺酰胺非对映异构体的制备尚不成熟,第二手性中心的影响尚未得到充分研究。我们报道了 Rh 催化的叔丁烷亚磺酰胺的不对称烯丙基化反应,合成了烯丙基叔丁亚磺酰胺的所有四种立体异构体。环己基取代的双恶唑啉膦配体的应用是控制区域选择性的关键。












































 京公网安备 11010802027423号
京公网安备 11010802027423号