Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diversified Synthesis of N-Benzoyl Piperidine Tetraoxane Analogues Using Molybdenum Trioxide as a Newer Catalyst and Its Antiplasmodial Activity
ACS Omega ( IF 3.7 ) Pub Date : 2024-07-11 , DOI: 10.1021/acsomega.4c01628 Arvind Kumar 1 , Drishti Agarwal 1 , Bhawana Sharma 1 , Rinkoo Devi Gupta 2 , Satish Kumar Awasthi 1
ACS Omega ( IF 3.7 ) Pub Date : 2024-07-11 , DOI: 10.1021/acsomega.4c01628 Arvind Kumar 1 , Drishti Agarwal 1 , Bhawana Sharma 1 , Rinkoo Devi Gupta 2 , Satish Kumar Awasthi 1
Affiliation
|
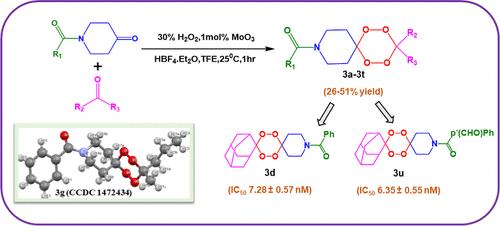
Molybdenum trioxide has been proven to be an efficient catalyst in synthesizing mixed N-benzoyl piperidine dispiro-1,2,4,5-tetraoxane analogues using a one-pot reaction. This catalyst facilitated the synthesis of 21 tetraoxanes using cyclic, acyclic, and aromatic ketones. The structure and methodology of this reaction have been validated by single-crystal X-ray analysis of compound ″3g″. The nature of dispiro-1,2,4,5-tetraoxane being synthesized has an impact on the overall yield of tetraoxanes such as symmetric dispiro-1,2,4,5-tetraoxanes ranging from 53 to 82%, while yields of N-benzoyl piperidine dispiro-1,2,4,5-tetraoxanes ranged from 26 to 51%. Additionally, the in vitro antiplasmodial activity of the newly developed tetraoxanes against Plasmodium falciparum was assessed, which exhibited significantly higher activity in the nanomolar range, with values ranging from 6.35 to 44.65 nM. Molecular docking studies revealed that newer tetraoxane derivatives bind to the active site of the falcipain-2 enzyme through H-bonding and hydrophobic contacts, which are the primary indicators of the effectiveness of the synthesized compounds. Findings suggest that these peculiar compounds may act as antiplasmodial agents, which spur further study and advancement in the battle against malaria.
中文翻译:

三氧化钼新型催化剂多样化合成N-苯甲酰哌啶四恶烷类似物及其抗疟原虫活性
三氧化钼已被证明是使用一锅反应合成混合N-苯甲酰哌啶二螺-1,2,4,5-四恶烷类似物的有效催化剂。该催化剂促进了使用环状、无环和芳香酮合成 21 种四恶烷。该反应的结构和方法已通过化合物“3g”的单晶X射线分析得到验证。所合成的二螺-1,2,4,5-四恶烷的性质对四恶烷的总收率有影响,例如对称二螺-1,2,4,5-四恶烷,范围为 53% 至 82%,而N的收率-苯甲酰哌啶二螺-1,2,4,5-四恶烷的范围为26%至51%。此外,还评估了新开发的四恶烷针对恶性疟原虫的体外抗疟原虫活性,其在纳摩尔范围内表现出显着更高的活性,值范围为 6.35 至 44.65 nM。分子对接研究表明,较新的四恶烷衍生物通过氢键和疏水接触与 falcipain-2 酶的活性位点结合,这是合成化合物有效性的主要指标。研究结果表明,这些奇特的化合物可能充当抗疟原虫剂,从而促进抗击疟疾的进一步研究和进展。
更新日期:2024-07-11
中文翻译:

三氧化钼新型催化剂多样化合成N-苯甲酰哌啶四恶烷类似物及其抗疟原虫活性
三氧化钼已被证明是使用一锅反应合成混合N-苯甲酰哌啶二螺-1,2,4,5-四恶烷类似物的有效催化剂。该催化剂促进了使用环状、无环和芳香酮合成 21 种四恶烷。该反应的结构和方法已通过化合物“3g”的单晶X射线分析得到验证。所合成的二螺-1,2,4,5-四恶烷的性质对四恶烷的总收率有影响,例如对称二螺-1,2,4,5-四恶烷,范围为 53% 至 82%,而N的收率-苯甲酰哌啶二螺-1,2,4,5-四恶烷的范围为26%至51%。此外,还评估了新开发的四恶烷针对恶性疟原虫的体外抗疟原虫活性,其在纳摩尔范围内表现出显着更高的活性,值范围为 6.35 至 44.65 nM。分子对接研究表明,较新的四恶烷衍生物通过氢键和疏水接触与 falcipain-2 酶的活性位点结合,这是合成化合物有效性的主要指标。研究结果表明,这些奇特的化合物可能充当抗疟原虫剂,从而促进抗击疟疾的进一步研究和进展。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号