Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis of homodimerization of the JNK scaffold protein JIP2 and its heterodimerization with JIP1
Structure ( IF 4.4 ) Pub Date : 2024-07-15 , DOI: 10.1016/j.str.2024.06.010 Laura Mariño Pérez 1 , Francesco S Ielasi 2 , Alexandra Lee 3 , Elise Delaforge 4 , Pauline Juyoux 4 , Maud Tengo 4 , Roger J Davis 3 , Andrés Palencia 2 , Malene Ringkjøbing Jensen 4
Structure ( IF 4.4 ) Pub Date : 2024-07-15 , DOI: 10.1016/j.str.2024.06.010 Laura Mariño Pérez 1 , Francesco S Ielasi 2 , Alexandra Lee 3 , Elise Delaforge 4 , Pauline Juyoux 4 , Maud Tengo 4 , Roger J Davis 3 , Andrés Palencia 2 , Malene Ringkjøbing Jensen 4
Affiliation
|
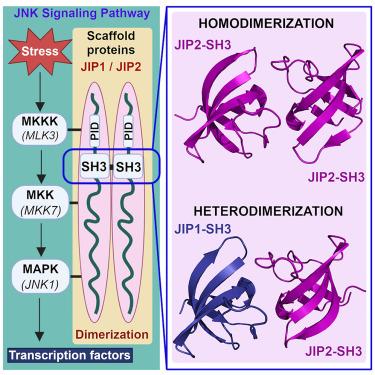
The scaffold proteins JIP1 and JIP2 intervene in the c-Jun N-terminal kinase (JNK) pathway to mediate signaling specificity by coordinating the simultaneous assembly of multiple kinases. Using NMR, we demonstrate that JIP1 and JIP2 heterodimerize via their SH3 domains with the affinity of heterodimerization being comparable to homodimerization. We present the high-resolution crystal structure of the JIP2-SH3 homodimer and the JIP1-JIP2-SH3 heterodimeric complex. The JIP2-SH3 structure reveals how charge differences in residues at its dimer interface lead to formation of compensatory hydrogen bonds and salt bridges, distinguishing it from JIP1-SH3. In the JIP1-JIP2-SH3 complex, structural features of each homodimer are employed to stabilize the heterodimer. Building on these insights, we identify key residues crucial for stabilizing the dimer of both JIP1 and JIP2. Through targeted mutations in cellulo , we demonstrate a functional role for the dimerization of the JIP1 and JIP2 scaffold proteins in activation of the JNK signaling pathway.
中文翻译:

JNK支架蛋白JIP2同二聚及其与JIP1异二聚的结构基础
支架蛋白 JIP1 和 JIP2 干预 c-Jun N 末端激酶 (JNK) 通路,通过协调多个激酶的同时组装来介导信号特异性。使用 NMR,我们证明 JIP1 和 JIP2 通过其 SH3 结构域异二聚化,异二聚化的亲和力与同二聚化相当。我们展示了 JIP2-SH3 同二聚体和 JIP1-JIP2-SH3 异二聚体复合物的高分辨率晶体结构。 JIP2-SH3结构揭示了其二聚体界面残基的电荷差异如何导致补偿性氢键和盐桥的形成,这与JIP1-SH3不同。在 JIP1-JIP2-SH3 复合物中,每个同二聚体的结构特征用于稳定异二聚体。基于这些见解,我们确定了对于稳定 JIP1 和 JIP2 二聚体至关重要的关键残基。通过纤维素中的靶向突变,我们证明了 JIP1 和 JIP2 支架蛋白二聚化在 JNK 信号通路激活中的功能作用。
更新日期:2024-07-15
中文翻译:

JNK支架蛋白JIP2同二聚及其与JIP1异二聚的结构基础
支架蛋白 JIP1 和 JIP2 干预 c-Jun N 末端激酶 (JNK) 通路,通过协调多个激酶的同时组装来介导信号特异性。使用 NMR,我们证明 JIP1 和 JIP2 通过其 SH3 结构域异二聚化,异二聚化的亲和力与同二聚化相当。我们展示了 JIP2-SH3 同二聚体和 JIP1-JIP2-SH3 异二聚体复合物的高分辨率晶体结构。 JIP2-SH3结构揭示了其二聚体界面残基的电荷差异如何导致补偿性氢键和盐桥的形成,这与JIP1-SH3不同。在 JIP1-JIP2-SH3 复合物中,每个同二聚体的结构特征用于稳定异二聚体。基于这些见解,我们确定了对于稳定 JIP1 和 JIP2 二聚体至关重要的关键残基。通过纤维素中的靶向突变,我们证明了 JIP1 和 JIP2 支架蛋白二聚化在 JNK 信号通路激活中的功能作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号