Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CO2 hydrogenation to formic acid promoted by acylthiourea-ruthenium complexes with ionic liquid
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-07-02 , DOI: 10.1016/j.jcat.2024.115633 L.C. Dresch , G.K. Rambor , K. Santos , J.A. Fetter , R. Cargnelutti , R.S. Oliboni , L. Colina-Vegas , O.L. Casagrande , R. Stieler
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-07-02 , DOI: 10.1016/j.jcat.2024.115633 L.C. Dresch , G.K. Rambor , K. Santos , J.A. Fetter , R. Cargnelutti , R.S. Oliboni , L. Colina-Vegas , O.L. Casagrande , R. Stieler
|
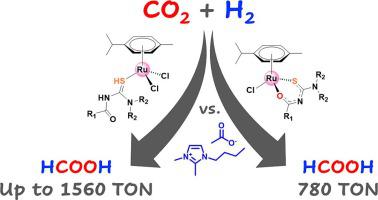
Herein we described the synthesis and characterization of a series of (η--cymene)ruthenium complexes based on acylthiourea ligands (), as well as their use as precatalysts for CO hydrogenation to formic acid (FA) utilizing 1-butyl-2,3-dimethylimidazolium acetate (BMMIm.OAc) ionic liquid as a reaction additive. The molecular structure of the new complexes were determined through elemental analysis, high-resolution mass spectrometry, infrared, H and C{H} NMR spectroscopy. Single crystal X-ray diffraction analyses of were also performed for the full characterization of the compounds. Theoretical calculations based on the Density Functional Theory were carried out to estimate the element contribution and energies of the Highest Occupied Molecular Orbital (HOMO) and Lowest Unoccupied Molecular Orbital (LUMO) of the complexes. Under similar reaction conditions (0.26 µmol of precatalyst; 0.66 mmol of BMMIm.OAc; 1 mL of solvent (95 % DMSO 5 % HO); 30 bar CO; 30 bar H; 70 °C) all complexes proved to be active for CO hydrogenation producing FA as the only product after 22 h of reaction with Turnover Numbers (TON) ranging from 780 to 1560. The results highlight the crucial role of the BMMIm.OAc ionic liquid in FA production, acting as a very mild base, buffering reaction medium, and stabilizing catalytically active species, boosting conversion and selectivity toward FA production. The investigation of CO hydrogenation using under different gas compositions suggested that the rate-determining step is dependent on H partial pressure. The monitoring of FA production over time using as precatalyst indicated that the catalytic system is more active at 100 °C initially, but more robust at 70 °C, achieving a maximum TON of 2434 after 36 h of reaction.
中文翻译:

酰基硫脲-钌配合物与离子液体促进CO2加氢制甲酸
在此,我们描述了一系列基于酰基硫脲配体 () 的 (η-伞花烃) 钌配合物的合成和表征,以及它们作为利用 1-丁基-2,3 进行 CO 加氢生成甲酸 (FA) 的预催化剂的用途-二甲基咪唑乙酸盐(BMMIm.OAc)离子液体作为反应添加剂。通过元素分析、高分辨率质谱、红外、H和C{H} NMR光谱确定了新配合物的分子结构。还进行了单晶 X 射线衍射分析,以全面表征化合物。基于密度泛函理论的理论计算估计了配合物最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)的元素贡献和能量。在类似的反应条件下(0.26 µmol 预催化剂;0.66 mmol BMMIm.OAc;1 mL 溶剂(95% DMSO 5% H2O);30 bar CO;30 bar H;70 °C),所有配合物均被证明对 CO 具有活性反应 22 小时后,氢化产生的 FA 是唯一产物,周转数 (TON) 范围为 780 至 1560。结果凸显了 BMMIm.OAc 离子液体在 FA 生产中的关键作用,充当非常温和的碱、缓冲反应介质,并稳定催化活性物质,提高 FA 生产的转化率和选择性。在不同气体组成下使用 CO 加氢的研究表明,速率决定步骤取决于 H 分压。使用预催化剂对 FA 产量随时间的监测表明,催化系统最初在 100°C 时活性更高,但在 70°C 时更稳定,反应 36 小时后达到最大 TON 2434。
更新日期:2024-07-02
中文翻译:

酰基硫脲-钌配合物与离子液体促进CO2加氢制甲酸
在此,我们描述了一系列基于酰基硫脲配体 () 的 (η-伞花烃) 钌配合物的合成和表征,以及它们作为利用 1-丁基-2,3 进行 CO 加氢生成甲酸 (FA) 的预催化剂的用途-二甲基咪唑乙酸盐(BMMIm.OAc)离子液体作为反应添加剂。通过元素分析、高分辨率质谱、红外、H和C{H} NMR光谱确定了新配合物的分子结构。还进行了单晶 X 射线衍射分析,以全面表征化合物。基于密度泛函理论的理论计算估计了配合物最高占据分子轨道(HOMO)和最低未占据分子轨道(LUMO)的元素贡献和能量。在类似的反应条件下(0.26 µmol 预催化剂;0.66 mmol BMMIm.OAc;1 mL 溶剂(95% DMSO 5% H2O);30 bar CO;30 bar H;70 °C),所有配合物均被证明对 CO 具有活性反应 22 小时后,氢化产生的 FA 是唯一产物,周转数 (TON) 范围为 780 至 1560。结果凸显了 BMMIm.OAc 离子液体在 FA 生产中的关键作用,充当非常温和的碱、缓冲反应介质,并稳定催化活性物质,提高 FA 生产的转化率和选择性。在不同气体组成下使用 CO 加氢的研究表明,速率决定步骤取决于 H 分压。使用预催化剂对 FA 产量随时间的监测表明,催化系统最初在 100°C 时活性更高,但在 70°C 时更稳定,反应 36 小时后达到最大 TON 2434。












































 京公网安备 11010802027423号
京公网安备 11010802027423号