当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surfactant-free CuPd nano-alloy for N2H4 oxidation-assisted H2 evolution and H2O2 detection
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2024-07-09 , DOI: 10.1016/j.jallcom.2024.175511 Xingwang Sun , Xinmei Liu , Wenglong Yang , Guobin Zhu
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2024-07-09 , DOI: 10.1016/j.jallcom.2024.175511 Xingwang Sun , Xinmei Liu , Wenglong Yang , Guobin Zhu
|
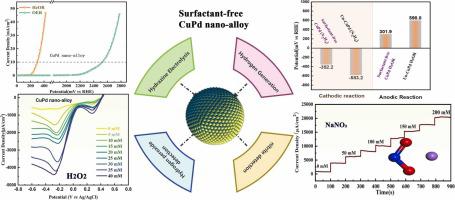
The utilization of hydrazine oxidation reaction (HzOR) with a lower thermodynamic oxidation potential has been regarded as an efficient strategy for H generation through electrolysis. However, a highly efficient and stable bifunctional electrocatalyst for overall hydrazine (NH) splitting was scarce. This work verified promising CuPd nano-alloy as a bifunctional electrocatalys for energy-efficient electrolytic hydrogen production, with hydrazine assistance. It is demonstrated that the CuPd nano-alloy exhibited enhanced electrocatalytic activity for both cathodic and anodic reactions compared to pure Cu and Pd nanocrystals, benefiting from its favorable electronic structure. Under 1.0 M KOH electrolyte with 0.1 M NH, the surfactant-free CuPd nano-alloy only required a potential of 0.648 V to achieve the NH splitting (OHzS). The surfactant-free surface maximized exposed active sites, which facilitated the adsorption and desorption of reactants, resulting in enhanced electrocatalytic activity. And working potential for HzOR by surfactant-free CuPd was only 50.4 % compared to the CuPd nano-alloy with surfactant. This work provided an efficient electrocatalyst for OHzS. The enhanced electro-catalytic performances resulting from the electronic structure of alloy and the surfactant-free interface effect have been thoroughly discussed and verified, which would be invaluable for the design of efficient electrocatalysts for H production.
中文翻译:

用于 N2H4 氧化辅助 H2 析出和 H2O2 检测的无表面活性剂 CuPd 纳米合金
利用具有较低热力学氧化电位的肼氧化反应(HzOR)被认为是电解产氢的有效策略。然而,用于整体肼(NH)分解的高效且稳定的双功能电催化剂却很稀缺。这项工作验证了 CuPd 纳米合金作为一种双功能电催化剂,在肼的辅助下,可用于高效节能的电解制氢。结果表明,与纯 Cu 和 Pd 纳米晶体相比,CuPd 纳米合金表现出增强的阴极和阳极反应电催化活性,这得益于其良好的电子结构。在含有0.1M NH的1.0M KOH电解液下,不含表面活性剂的CuPd纳米合金仅需要0.648V的电位即可实现NH分解(OHzS)。无表面活性剂的表面最大限度地暴露了活性位点,有利于反应物的吸附和解吸,从而增强了电催化活性。与含表面活性剂的 CuPd 纳米合金相比,不含表面活性剂的 CuPd 的 HzOR 工作潜力仅为 50.4%。这项工作为 OHzS 提供了一种高效的电催化剂。合金的电子结构和无表面活性剂界面效应带来的增强电催化性能已经得到了深入的讨论和验证,这对于设计高效的产氢电催化剂具有不可估量的价值。
更新日期:2024-07-09
中文翻译:

用于 N2H4 氧化辅助 H2 析出和 H2O2 检测的无表面活性剂 CuPd 纳米合金
利用具有较低热力学氧化电位的肼氧化反应(HzOR)被认为是电解产氢的有效策略。然而,用于整体肼(NH)分解的高效且稳定的双功能电催化剂却很稀缺。这项工作验证了 CuPd 纳米合金作为一种双功能电催化剂,在肼的辅助下,可用于高效节能的电解制氢。结果表明,与纯 Cu 和 Pd 纳米晶体相比,CuPd 纳米合金表现出增强的阴极和阳极反应电催化活性,这得益于其良好的电子结构。在含有0.1M NH的1.0M KOH电解液下,不含表面活性剂的CuPd纳米合金仅需要0.648V的电位即可实现NH分解(OHzS)。无表面活性剂的表面最大限度地暴露了活性位点,有利于反应物的吸附和解吸,从而增强了电催化活性。与含表面活性剂的 CuPd 纳米合金相比,不含表面活性剂的 CuPd 的 HzOR 工作潜力仅为 50.4%。这项工作为 OHzS 提供了一种高效的电催化剂。合金的电子结构和无表面活性剂界面效应带来的增强电催化性能已经得到了深入的讨论和验证,这对于设计高效的产氢电催化剂具有不可估量的价值。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号