当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Construction of Ethenyl-Substituted Acyclic Quaternary Stereocenters at the α-Position of Carbonyl Surrogates via Stereoselective Sulfonylvinylation–Reductive Desulfonylation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-07-12 , DOI: 10.1021/acs.joc.4c01235 Nuermaimaiti Yisimayili 1 , Chong-Dao Lu 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-07-12 , DOI: 10.1021/acs.joc.4c01235 Nuermaimaiti Yisimayili 1 , Chong-Dao Lu 1, 2
Affiliation
|
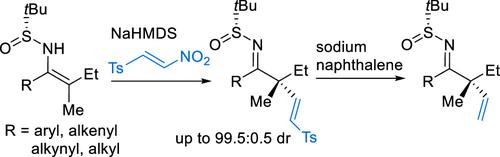
N-Sulfinyl metalloenamines, derived from geometry-defined β,β-disubstituted enesulfinamides, undergo conjugate addition–elimination reaction with β-tosyl nitroolefin to afford α-sulfonylvinylated ketimines with high stereocontrol. Further desulfonylation using sodium naphthalenide gives carbonyl surrogates bearing a less-accessible acyclic quaternary α-stereocenters substituted with an ethenyl group and two sterically and electronically similar groups (e.g., methyl and ethyl). Synthetic application of the described protocol was demonstrated by enantioselective synthesis of (S)-bakuchiol.
中文翻译:

通过立体选择性磺酰乙烯基化-还原脱磺酰化在羰基替代物的α位上不对称构建乙烯基取代的无环四元立构中心
N-亚磺酰金属烯胺衍生自几何定义的 β,β-二取代烯亚磺酰胺,与 β-甲苯磺酰硝基烯烃发生共轭加成-消除反应,得到具有高立体控制的 α-磺酰乙烯基化酮亚胺。使用萘化钠进一步脱磺酰化得到带有不易接近的无环四元α-立构中心的羰基替代物,该中心被乙烯基和两个空间和电子相似的基团(例如甲基和乙基)取代。通过 ( S )-bakuchiol 的对映选择性合成证明了所描述协议的合成应用。
更新日期:2024-07-12
中文翻译:

通过立体选择性磺酰乙烯基化-还原脱磺酰化在羰基替代物的α位上不对称构建乙烯基取代的无环四元立构中心
N-亚磺酰金属烯胺衍生自几何定义的 β,β-二取代烯亚磺酰胺,与 β-甲苯磺酰硝基烯烃发生共轭加成-消除反应,得到具有高立体控制的 α-磺酰乙烯基化酮亚胺。使用萘化钠进一步脱磺酰化得到带有不易接近的无环四元α-立构中心的羰基替代物,该中心被乙烯基和两个空间和电子相似的基团(例如甲基和乙基)取代。通过 ( S )-bakuchiol 的对映选择性合成证明了所描述协议的合成应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号