当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Dichloramine Hydrolysis in Membrane Desalination Permeate: Mechanistic Insights and Implications for Oxidative Capacity in Potable Reuse Applications
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-07-12 , DOI: 10.1021/acs.est.4c04547 Liang Wu 1, 2 , Sitao Liu 2 , Haizhou Liu 1, 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-07-12 , DOI: 10.1021/acs.est.4c04547 Liang Wu 1, 2 , Sitao Liu 2 , Haizhou Liu 1, 2
Affiliation
|
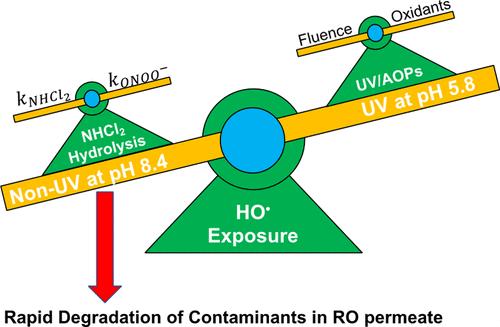
Dichloramine (NHCl2) naturally exists in reverse osmosis (RO) permeate due to its application as an antifouling chemical in membrane-based potable reuse treatment. This study investigated mechanisms of background NHCl2 hydrolysis associated with the generation of oxidative radical species in RO permeate, established a kinetic model to predict the oxidative capacity, and examined its removal efficiency on trace organic contaminants in potable reuse. Results showed that NHCl2 hydrolysis generated transient peroxynitrite (ONOO–) and subsequently dissociated into hydroxyl radical (HO•). The maximal HO• exposure was observed at an RO permeate pH of 8.4, higher than that from typical ultraviolet (UV)-based advanced oxidation processes. The HO• exposure during NHCl2 hydrolysis also peaked at a NH2Cl-to-NHCl2 molar ratio of 1:1. The oxidative capacity rapidly degraded 1,4-dioxane, carbamazepine, atenolol, and sulfamethoxazole in RO permeate. Furthermore, background elevated carbonate in fresh RO permeate can convert HO• to carbonate radical (CO3•–). Aeration of the RO permeate removed total carbonate, significantly increased HO• exposure, and enhanced the degradation kinetics of trace organic contaminants. The kinetic model of NHCl2 hydrolysis predicted well the degradation of contaminants in RO permeate. This study provides new mechanistic insights into NHCl2 hydrolysis that contributes to the oxidative degradation of trace organic contaminants in potable reuse systems.
中文翻译:

膜淡化渗透物中的二氯胺水解:饮用水再利用应用中氧化能力的机理见解和影响
二氯胺 (NHCl 2 ) 天然存在于反渗透 (RO) 渗透液中,因为它在基于膜的饮用水回用处理中用作防污化学品。本研究研究了背景 NHCl 2 水解与 RO 渗透液中氧化自由基物质生成相关的机制,建立了预测氧化能力的动力学模型,并检验了其对饮用水回用中痕量有机污染物的去除效率。结果表明,NH4Cl 2 水解产生瞬时过氧亚硝酸盐(ONOO – ),随后解离为羟基自由基(HO • )。在 RO 渗透液 pH 值为 8.4 时观察到最大 H2O • 暴露,高于典型的基于紫外线 (UV) 的高级氧化工艺。 NHCl 2 水解过程中 H2O • 暴露也在 NH 2 Cl-to-NHCl 2 摩尔比为 1:1 时达到峰值。氧化能力快速降解 RO 渗透液中的 1,4-二恶烷、卡马西平、阿替洛尔和磺胺甲恶唑。此外,新鲜 RO 渗透物中背景升高的碳酸盐可以将 H2O • 转化为碳酸盐自由基 (CO 3 •– )。 RO 渗透物的曝气去除了总碳酸盐,显着增加了 H2O • 暴露,并增强了痕量有机污染物的降解动力学。 NHCl 2 水解动力学模型很好地预测了RO渗透水中污染物的降解。这项研究为 NHCl 2 水解提供了新的机制见解,该水解有助于饮用水再利用系统中痕量有机污染物的氧化降解。
更新日期:2024-07-12
中文翻译:

膜淡化渗透物中的二氯胺水解:饮用水再利用应用中氧化能力的机理见解和影响
二氯胺 (NHCl 2 ) 天然存在于反渗透 (RO) 渗透液中,因为它在基于膜的饮用水回用处理中用作防污化学品。本研究研究了背景 NHCl 2 水解与 RO 渗透液中氧化自由基物质生成相关的机制,建立了预测氧化能力的动力学模型,并检验了其对饮用水回用中痕量有机污染物的去除效率。结果表明,NH4Cl 2 水解产生瞬时过氧亚硝酸盐(ONOO – ),随后解离为羟基自由基(HO • )。在 RO 渗透液 pH 值为 8.4 时观察到最大 H2O • 暴露,高于典型的基于紫外线 (UV) 的高级氧化工艺。 NHCl 2 水解过程中 H2O • 暴露也在 NH 2 Cl-to-NHCl 2 摩尔比为 1:1 时达到峰值。氧化能力快速降解 RO 渗透液中的 1,4-二恶烷、卡马西平、阿替洛尔和磺胺甲恶唑。此外,新鲜 RO 渗透物中背景升高的碳酸盐可以将 H2O • 转化为碳酸盐自由基 (CO 3 •– )。 RO 渗透物的曝气去除了总碳酸盐,显着增加了 H2O • 暴露,并增强了痕量有机污染物的降解动力学。 NHCl 2 水解动力学模型很好地预测了RO渗透水中污染物的降解。这项研究为 NHCl 2 水解提供了新的机制见解,该水解有助于饮用水再利用系统中痕量有机污染物的氧化降解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号