Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Otilonium Bromide Exhibits Potent Antifungal Effects by Blocking Ergosterol Plasma Membrane Localization and Triggering Cytotoxic Autophagy in Candida Albicans
Advanced Science ( IF 14.3 ) Pub Date : 2024-07-12 , DOI: 10.1002/advs.202406473 Cheng Zhen 1 , Li Wang 1 , Yanru Feng 1 , Malcolm Whiteway 2 , Sijin Hang 1 , Jinhua Yu 1 , Hui Lu 1 , Yuanying Jiang 1
Advanced Science ( IF 14.3 ) Pub Date : 2024-07-12 , DOI: 10.1002/advs.202406473 Cheng Zhen 1 , Li Wang 1 , Yanru Feng 1 , Malcolm Whiteway 2 , Sijin Hang 1 , Jinhua Yu 1 , Hui Lu 1 , Yuanying Jiang 1
Affiliation
|
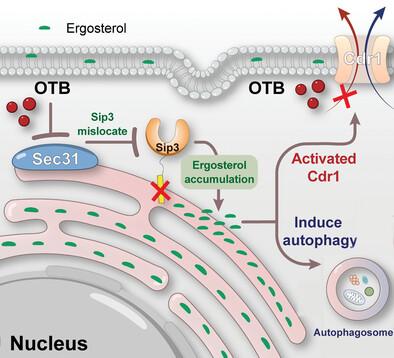
Candidiasis, which presents a substantial risk to human well-being, is frequently treated with azoles. However, drug-drug interactions caused by azoles inhibiting the human CYP3A4 enzyme, together with increasing resistance of Candida species to azoles, represent serious issues with this class of drug, making it imperative to develop innovative antifungal drugs to tackle this growing clinical challenge. A drug repurposing approach is used to examine a library of Food and Drug Administration (FDA)-approved drugs, ultimately identifying otilonium bromide (OTB) as an exceptionally encouraging antifungal agent. Mechanistically, OTB impairs vesicle-mediated trafficking by targeting Sec31, thereby impeding the plasma membrane (PM) localization of the ergosterol transporters, such as Sip3. Consequently, OTB obstructs the movement of ergosterol across membranes and triggers cytotoxic autophagy. It is noteworthy that C. albicans encounters challenges in developing resistance to OTB because it is not a substrate for drug transporters. This study opens a new door for antifungal therapy, wherein OTB disrupts ergosterol subcellular distribution and induces cytotoxic autophagy. Additionally, it circumvents the hepatotoxicity associated with azole-mediated liver enzyme inhibition and avoids export-mediated drug resistance in C. albicans.
中文翻译:

奥替溴铵通过阻断白色念珠菌的麦角甾醇质膜定位和触发细胞毒性自噬而表现出有效的抗真菌作用
念珠菌病对人类健康构成重大风险,经常用唑类药物治疗。然而,由唑类药物抑制人类 CYP3A4 酶引起的药物相互作用,以及念珠菌属对唑类药物耐药性的增加,代表了此类药物的严重问题,因此必须开发创新的抗真菌药物来应对这一日益严峻的临床挑战。药物再利用方法用于检查美国食品和药物管理局 (FDA) 批准的药物库,最终确定奥替溴铵 (OTB) 是一种非常令人鼓舞的抗真菌药物。从机制上讲,OTB 通过靶向 Sec31 损害囊泡介导的运输,从而阻碍麦角甾醇转运蛋白(例如 Sip3)的质膜(PM)定位。因此,OTB 阻碍麦角甾醇跨膜运动并引发细胞毒性自噬。值得注意的是,白色念珠菌在产生 OTB 耐药性方面遇到了挑战,因为它不是药物转运蛋白的底物。这项研究为抗真菌治疗打开了一扇新的大门,其中 OTB 会破坏麦角甾醇的亚细胞分布并诱导细胞毒性自噬。此外,它还避免了与唑介导的肝酶抑制相关的肝毒性,并避免了白色念珠菌中输出介导的耐药性。
更新日期:2024-07-12
中文翻译:

奥替溴铵通过阻断白色念珠菌的麦角甾醇质膜定位和触发细胞毒性自噬而表现出有效的抗真菌作用
念珠菌病对人类健康构成重大风险,经常用唑类药物治疗。然而,由唑类药物抑制人类 CYP3A4 酶引起的药物相互作用,以及念珠菌属对唑类药物耐药性的增加,代表了此类药物的严重问题,因此必须开发创新的抗真菌药物来应对这一日益严峻的临床挑战。药物再利用方法用于检查美国食品和药物管理局 (FDA) 批准的药物库,最终确定奥替溴铵 (OTB) 是一种非常令人鼓舞的抗真菌药物。从机制上讲,OTB 通过靶向 Sec31 损害囊泡介导的运输,从而阻碍麦角甾醇转运蛋白(例如 Sip3)的质膜(PM)定位。因此,OTB 阻碍麦角甾醇跨膜运动并引发细胞毒性自噬。值得注意的是,白色念珠菌在产生 OTB 耐药性方面遇到了挑战,因为它不是药物转运蛋白的底物。这项研究为抗真菌治疗打开了一扇新的大门,其中 OTB 会破坏麦角甾醇的亚细胞分布并诱导细胞毒性自噬。此外,它还避免了与唑介导的肝酶抑制相关的肝毒性,并避免了白色念珠菌中输出介导的耐药性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号