当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-Neutral Vicarious-Type Nucleophilic Amination of Heterocyclic N-Oxides with Organic Azides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-07-10 , DOI: 10.1002/adsc.202400669 Jeonghyun Min 1 , Sujin Min 1 , Eunjae Chung 1 , Kyeongwon Moon 1 , Hyung Sik Kim 1 , Taejoo Jeong 1 , AMITAVA RAKSHIT 1 , Pargat Singh 1 , Jung Su Park 2 , In Su Kim 1
中文翻译:

杂环氮氧化物与有机叠氮化物的氧化还原中性替代型亲核胺化
更新日期:2024-07-10
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-07-10 , DOI: 10.1002/adsc.202400669 Jeonghyun Min 1 , Sujin Min 1 , Eunjae Chung 1 , Kyeongwon Moon 1 , Hyung Sik Kim 1 , Taejoo Jeong 1 , AMITAVA RAKSHIT 1 , Pargat Singh 1 , Jung Su Park 2 , In Su Kim 1
Affiliation
|
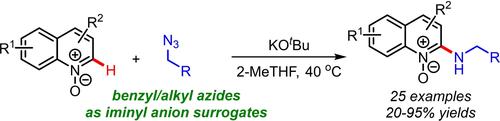
Site-selective amination of N-heterocycles is an interesting topic in organic synthesis and drug discovery. We herein present a method for the vicarious-type nucleophilic amination of azine N-oxides and cyclic iminoamides using iminyl anions, readily generated from organic azides under basic conditions. The plausible reaction mechanism involving nucleophilic aromatic substitution by iminyl anions is elucidated by a series of mechanistic investigations.
中文翻译:

杂环氮氧化物与有机叠氮化物的氧化还原中性替代型亲核胺化
N-杂环的位点选择性胺化是有机合成和药物发现中的一个有趣的话题。我们在此提出了一种使用亚氨基阴离子对吖嗪N-氧化物和环状亚氨基酰胺进行替代型亲核胺化的方法,亚氨基阴离子很容易在碱性条件下由有机叠氮化物产生。通过一系列机理研究阐明了涉及亚胺基阴离子亲核芳族取代的合理反应机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号