当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-free synthesis of hydrazino-containing glycine derivatives via a diaziridine in situ formation/ring-opening cascade
Green Chemistry ( IF 9.3 ) Pub Date : 2024-07-12 , DOI: 10.1039/d4gc02565b Chang-Long Rong 1 , Qiang-Qiang Li 1 , Jun Xuan 1
Green Chemistry ( IF 9.3 ) Pub Date : 2024-07-12 , DOI: 10.1039/d4gc02565b Chang-Long Rong 1 , Qiang-Qiang Li 1 , Jun Xuan 1
Affiliation
|
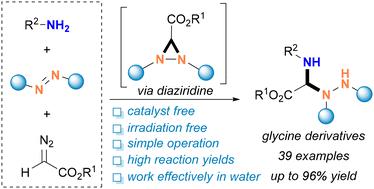
Glycine derivatives are prevalent structural motifs that can be easily found in many bioactive molecules and natural isolates. To date, chemical synthesis of hydrazino-containing glycine derivatives has mainly focused on the utilization of metal catalysts or light-irradiation methods. Herein, we report a three-component reaction of amines, azodicarboxylates, and diazoalkanes under green and sustainable reaction conditions, leading to a wide range of hydrazino-containing glycine derivatives in good to excellent yields. Compared to the reported methods, the as-developed method does not require catalysts or light irradiation. The success of the scale-up reaction, synthetic transformation of the formed glycine products, and the direct aqueous media synthesis further rendered the method attractive and valuable. Control experiments together with detailed mechanism studies revealed that the in situ formed diaziridines are the key reaction intermediates.
中文翻译:

通过二氮丙啶原位形成/开环级联无催化剂合成含肼基甘氨酸衍生物
甘氨酸衍生物是常见的结构基序,可以在许多生物活性分子和天然分离物中轻松找到。迄今为止,含肼基甘氨酸衍生物的化学合成主要集中在利用金属催化剂或光照射方法。在此,我们报道了胺、偶氮二甲酸酯和重氮烷在绿色和可持续反应条件下的三组分反应,以良好至优异的产率产生了各种含肼的甘氨酸衍生物。与报道的方法相比,所开发的方法不需要催化剂或光照射。放大反应、所形成的甘氨酸产物的合成转化以及直接水介质合成的成功进一步使得该方法具有吸引力和价值。对照实验和详细的机理研究表明,原位形成的二氮丙啶是关键的反应中间体。
更新日期:2024-07-12
中文翻译:

通过二氮丙啶原位形成/开环级联无催化剂合成含肼基甘氨酸衍生物
甘氨酸衍生物是常见的结构基序,可以在许多生物活性分子和天然分离物中轻松找到。迄今为止,含肼基甘氨酸衍生物的化学合成主要集中在利用金属催化剂或光照射方法。在此,我们报道了胺、偶氮二甲酸酯和重氮烷在绿色和可持续反应条件下的三组分反应,以良好至优异的产率产生了各种含肼的甘氨酸衍生物。与报道的方法相比,所开发的方法不需要催化剂或光照射。放大反应、所形成的甘氨酸产物的合成转化以及直接水介质合成的成功进一步使得该方法具有吸引力和价值。对照实验和详细的机理研究表明,原位形成的二氮丙啶是关键的反应中间体。































 京公网安备 11010802027423号
京公网安备 11010802027423号