当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Importance of the catalyst–water Coulomb interaction for oxygen reduction reaction kinetics
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-07-12 , DOI: 10.1039/d4ee01760a Teng Liu 1 , Yinghe Zhao 1 , Tianyou Zhai 1
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-07-12 , DOI: 10.1039/d4ee01760a Teng Liu 1 , Yinghe Zhao 1 , Tianyou Zhai 1
Affiliation
|
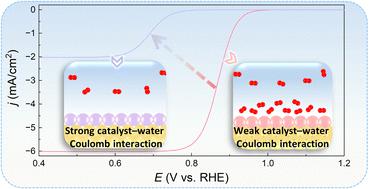
We identify an important new factor affecting oxygen reduction reaction (ORR) kinetics (i.e., the catalyst–water Coulomb interaction). A stronger Coulomb interaction leads to a stronger water wall at the catalyst/water interface and thus renders the contact between catalytic sites and O2 molecules more difficult, thereby resulting in more sluggish ORR kinetics. The difference in ORR kinetics can reach 10 000-fold between the catalyst with the strong Coulomb interaction and that with the weak Coulomb interaction. Additionally, we reveal an important (yet previously overlooked) prerequisite for a material to be an ORR electrocatalyst comparable to Pt: the atomic charge of catalytic sites should be between −1.0 e and 0.9 e (to circumvent the strong Coulomb interaction). This finding explains well recent experimental results showing that transition metal oxides based on Fe, Y, Ni, Mn, and La have intrinsically limited ORR kinetics (H. Li, et al., Nat. Catal., 2021, 4, 463–468.). The successful explanation further substantiates the plausibility of the finding. Furthermore, we demonstrate that the catalyst–water Coulomb interaction also has a similar effect on the other electrocatalytic reactions consuming small nonpolar molecules, such as the hydrogen oxidation reaction and the nitrogen reduction reaction.
中文翻译:

催化剂-水库仑相互作用对于氧还原反应动力学的重要性
我们确定了影响氧还原反应(ORR)动力学(即催化剂-水库仑相互作用)的一个重要的新因素。更强的库仑相互作用会导致催化剂/水界面处的水壁更强,从而使催化位点与 O 2 分子之间的接触更加困难,从而导致 ORR 动力学更加缓慢。强库仑相互作用的催化剂与弱库仑相互作用的催化剂之间的ORR动力学差异可达10 000倍。此外,我们还揭示了材料成为与 Pt 相当的 ORR 电催化剂的一个重要(但之前被忽视)的先决条件:催化位点的原子电荷应在 -1.0 e 至 0.9 e 之间(以避免强库仑相互作用)。这一发现很好地解释了最近的实验结果,表明基于 Fe、Y、Ni、Mn 和 La 的过渡金属氧化物本质上限制了 ORR 动力学 (H. Li, et al., Nat. Catal., 2021, 4, 463–468 .)。成功的解释进一步证实了这一发现的合理性。此外,我们证明催化剂-水库仑相互作用对消耗小非极性分子的其他电催化反应也有类似的影响,例如氢氧化反应和氮还原反应。
更新日期:2024-07-12
中文翻译:

催化剂-水库仑相互作用对于氧还原反应动力学的重要性
我们确定了影响氧还原反应(ORR)动力学(即催化剂-水库仑相互作用)的一个重要的新因素。更强的库仑相互作用会导致催化剂/水界面处的水壁更强,从而使催化位点与 O 2 分子之间的接触更加困难,从而导致 ORR 动力学更加缓慢。强库仑相互作用的催化剂与弱库仑相互作用的催化剂之间的ORR动力学差异可达10 000倍。此外,我们还揭示了材料成为与 Pt 相当的 ORR 电催化剂的一个重要(但之前被忽视)的先决条件:催化位点的原子电荷应在 -1.0 e 至 0.9 e 之间(以避免强库仑相互作用)。这一发现很好地解释了最近的实验结果,表明基于 Fe、Y、Ni、Mn 和 La 的过渡金属氧化物本质上限制了 ORR 动力学 (H. Li, et al., Nat. Catal., 2021, 4, 463–468 .)。成功的解释进一步证实了这一发现的合理性。此外,我们证明催化剂-水库仑相互作用对消耗小非极性分子的其他电催化反应也有类似的影响,例如氢氧化反应和氮还原反应。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号