当前位置:
X-MOL 学术
›
J. Am. Soc. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing High-order Protein Complexes Using Native Mass Spectrometry and Hydrogen/Deuterium Exchange Mass Spectrometry: A Case Study Using Fresh and Commercial Hemoglobin Samples
Journal of the American Society for Mass Spectrometry ( IF 3.1 ) Pub Date : 2024-07-03 , DOI: 10.1021/jasms.4c00201 T.-Y Lui 1 , Xiangfeng Chen 1, 2 , Danna Hu 1 , T.-W. Dominic Chan 1
Journal of the American Society for Mass Spectrometry ( IF 3.1 ) Pub Date : 2024-07-03 , DOI: 10.1021/jasms.4c00201 T.-Y Lui 1 , Xiangfeng Chen 1, 2 , Danna Hu 1 , T.-W. Dominic Chan 1
Affiliation
|
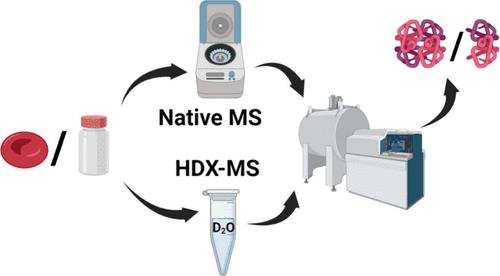
Native mass spectrometry (MS) analysis of protein complexes is highly susceptible to matrix effect, and addressing this predicament using buffer exchange is a common approach. Nevertheless, optimization of the buffer exchange protocol is not trivial. With the use of hemoglobin (Hb) as the model entity, it was discovered that the native mass spectrum of protein assembly is highly dependent on the buffer-exchange protocol. Given the dependence of native MS on the purification protocol, this work attempts to use hydrogen/deuterium exchange mass spectrometry (HDX-MS) for comparative studies of hemoglobin complexes in untreated fresh and commercial samples. The information obtained from the HDX study was found to correlate well with the native mass spectrometry analysis of the properly buffer-exchanged Hb samples. Both native MS and HDX-MS showed that the fresh Hb sample has retained the expected tetrameric structure, whereas the commercial Hb has largely been denatured to the dimeric form. These findings prove the complementarity of native MS and HDX-MS in the analysis of high-order protein complexes and stress the necessity to validate the integrity of the high-order structures of the proteins prior to the use of the protein samples for other biomedical studies.
中文翻译:

使用天然质谱和氢/氘交换质谱探测高阶蛋白质复合物:使用新鲜和商业血红蛋白样品的案例研究
蛋白质复合物的天然质谱 (MS) 分析非常容易受到基质效应的影响,使用缓冲液交换来解决这一困境是一种常见的方法。然而,缓冲液交换协议的优化并非易事。使用血红蛋白 (Hb) 作为模型实体,发现蛋白质组装的天然质谱高度依赖于缓冲液交换方案。鉴于天然 MS 对纯化方案的依赖性,本工作尝试使用氢/氘交换质谱 (HDX-MS) 对未经处理的新鲜样品和商业样品中的血红蛋白复合物进行比较研究。发现从 HDX 研究中获得的信息与正确缓冲液交换的 Hb 样品的天然质谱分析有很好的相关性。天然 MS 和 HDX-MS 均表明,新鲜 Hb 样品保留了预期的四聚体结构,而商业 Hb 很大程度上已变性为二聚体形式。这些发现证明了天然 MS 和 HDX-MS 在分析高阶蛋白质复合物中的互补性,并强调在将蛋白质样品用于其他生物医学研究之前验证蛋白质高阶结构的完整性的必要性。
更新日期:2024-07-03
中文翻译:

使用天然质谱和氢/氘交换质谱探测高阶蛋白质复合物:使用新鲜和商业血红蛋白样品的案例研究
蛋白质复合物的天然质谱 (MS) 分析非常容易受到基质效应的影响,使用缓冲液交换来解决这一困境是一种常见的方法。然而,缓冲液交换协议的优化并非易事。使用血红蛋白 (Hb) 作为模型实体,发现蛋白质组装的天然质谱高度依赖于缓冲液交换方案。鉴于天然 MS 对纯化方案的依赖性,本工作尝试使用氢/氘交换质谱 (HDX-MS) 对未经处理的新鲜样品和商业样品中的血红蛋白复合物进行比较研究。发现从 HDX 研究中获得的信息与正确缓冲液交换的 Hb 样品的天然质谱分析有很好的相关性。天然 MS 和 HDX-MS 均表明,新鲜 Hb 样品保留了预期的四聚体结构,而商业 Hb 很大程度上已变性为二聚体形式。这些发现证明了天然 MS 和 HDX-MS 在分析高阶蛋白质复合物中的互补性,并强调在将蛋白质样品用于其他生物医学研究之前验证蛋白质高阶结构的完整性的必要性。














































 京公网安备 11010802027423号
京公网安备 11010802027423号