当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
3-Substituted 6-Azabicyclo[3.1.1]heptanes: Nonclassical Piperidine Isosteres for Drug Discovery
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-07-11 , DOI: 10.1021/acs.joc.4c00326 Anton V Chernykh 1, 2, 3 , Bohdan V Vashchenko 1, 3 , Svitlana V Shishkina 2, 4 , Dmytro M Volochnyuk 1, 2, 3 , Oleksandr O Grygorenko 1, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-07-11 , DOI: 10.1021/acs.joc.4c00326 Anton V Chernykh 1, 2, 3 , Bohdan V Vashchenko 1, 3 , Svitlana V Shishkina 2, 4 , Dmytro M Volochnyuk 1, 2, 3 , Oleksandr O Grygorenko 1, 3
Affiliation
|
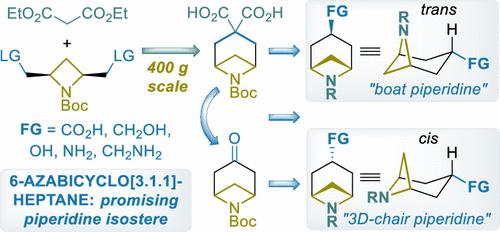
Advanced analogs of piperidine and smaller homologues of tropane─3-substituted 6-azabicyclo[3.1.1]heptanes─were synthesized on a large scale using readily available bulk reagents. The key step of the approach involved the double alkylation reaction of malonate with cis-2,4-bis(mesyloxymethyl)azetidine-1-carboxylate, in turn easily prepared on up to 1 kg scale. After hydrolysis, N-Boc-6-azabicyclo[3.1.1]heptane-3,3-dicarboxylic acid was obtained (up to 400 g in a single run), which was used as a common intermediate for the preparation of all the title building blocks. In particular, Pb(OAc)4-mediated oxidative decarboxylation of this intermediate gave 2,6-methanopiperidone derivative (up to 400 g scale), while monodecarboxylation gave N-Boc-6-azabicyclo[3.1.1]heptane-3-carboxylic acids as an easily separatable mixture of cis and trans diastereomers (up to 100 g scale). Further functional group transformations gave diastereopure cis- and trans-N-Boc-monoprotected diamines and amino alcohols. Molecular structure analysis using exit vector parameters (EVP) revealed that cis isomers of 3-substituted 6-azabicyclo[3.1.1]heptanes are three-dimensional analogs of common 1,4-disubstituted piperidine chair conformer, whereas trans isomers can be considered as unusual “boat” piperidines.
中文翻译:

3-取代的 6-氮杂双环[3.1.1]庚烷:用于药物发现的非经典哌啶电子等排体
使用现成的批量试剂大规模合成了哌啶的高级类似物和托烷的较小同系物 — 3-取代的 6-氮杂双环[3.1.1]庚烷。该方法的关键步骤涉及丙二酸与顺式-2,4-双(甲磺氧基甲基)氮杂环丁烷-1-羧酸酯的双烷基化反应,进而可以轻松制备高达1千克的规模。水解后,得到N -Boc-6-氮杂双环[3.1.1]庚烷-3,3-二甲酸(单次最多400 g),将其用作制备所有标题的通用中间体积木。特别是,该中间体的 Pb(OAc) 4介导的氧化脱羧得到 2,6-亚甲基哌啶酮衍生物(高达 400 g 规模),而单脱羧得到N -Boc-6-氮杂双环[3.1.1]庚烷-3-羧基酸作为顺式和反式非对映异构体的易于分离的混合物(最多 100 克规模)。进一步的官能团转化得到非对映纯的顺式和反式N -Boc-单保护的二胺和氨基醇。使用退出矢量参数(EVP)的分子结构分析表明,3-取代的6-氮杂双环[3.1.1]庚烷的顺式异构体是常见1,4-二取代哌啶椅构象异构体的三维类似物,而反式异构体可以被认为是不寻常的“船”哌啶。
更新日期:2024-07-11
中文翻译:

3-取代的 6-氮杂双环[3.1.1]庚烷:用于药物发现的非经典哌啶电子等排体
使用现成的批量试剂大规模合成了哌啶的高级类似物和托烷的较小同系物 — 3-取代的 6-氮杂双环[3.1.1]庚烷。该方法的关键步骤涉及丙二酸与顺式-2,4-双(甲磺氧基甲基)氮杂环丁烷-1-羧酸酯的双烷基化反应,进而可以轻松制备高达1千克的规模。水解后,得到N -Boc-6-氮杂双环[3.1.1]庚烷-3,3-二甲酸(单次最多400 g),将其用作制备所有标题的通用中间体积木。特别是,该中间体的 Pb(OAc) 4介导的氧化脱羧得到 2,6-亚甲基哌啶酮衍生物(高达 400 g 规模),而单脱羧得到N -Boc-6-氮杂双环[3.1.1]庚烷-3-羧基酸作为顺式和反式非对映异构体的易于分离的混合物(最多 100 克规模)。进一步的官能团转化得到非对映纯的顺式和反式N -Boc-单保护的二胺和氨基醇。使用退出矢量参数(EVP)的分子结构分析表明,3-取代的6-氮杂双环[3.1.1]庚烷的顺式异构体是常见1,4-二取代哌啶椅构象异构体的三维类似物,而反式异构体可以被认为是不寻常的“船”哌啶。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号