当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of peracetic acid by electrodes using biogenic electrons: A novel energy- and catalyst-free process to eliminate pharmaceuticals
Water Research ( IF 11.4 ) Pub Date : 2024-07-08 , DOI: 10.1016/j.watres.2024.122065 Rusen Zou 1 , Wenqiang Yang 2 , Babak Rezaei 3 , Kai Tang 1 , Kuangxin Guo 1 , Pingping Zhang 4 , Stephan Sylvest Keller 3 , Henrik Rasmus Andersen 1 , Yifeng Zhang 1
Water Research ( IF 11.4 ) Pub Date : 2024-07-08 , DOI: 10.1016/j.watres.2024.122065 Rusen Zou 1 , Wenqiang Yang 2 , Babak Rezaei 3 , Kai Tang 1 , Kuangxin Guo 1 , Pingping Zhang 4 , Stephan Sylvest Keller 3 , Henrik Rasmus Andersen 1 , Yifeng Zhang 1
Affiliation
|
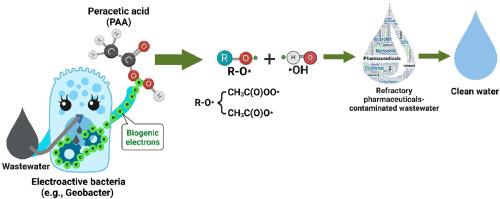
Peracetic acid (PAA) has received increasing attention as an alternative oxidant for wastewater treatment. However, existing processes for PAA activation to generate reactive species typically require external energy input (e.g., electrically and UV-mediated activation) or catalysts (e.g., Co2+ ), inevitably increasing treatment costs or introducing potential new contaminants that necessitate additional removal. In this work, we developed a catalyst-free, self-sustaining bioelectrochemical approach within a two-chamber bioelectrochemical system (BES), where a cathode electrode in-situ activates PAA using renewable biogenic electrons generated by anodic exoelectrogens (e.g., Geobacter) degrading biodegradable organic matter (e.g., acetic acid) in wastewater at the anode. This innovative BES-PAA technique achieved 98 % and 81 % removal of 2 µM sulfamethoxazole (SMX) in two hours at pH 2 (cation exchange membrane) and pH 6 (bipolar membrane) using 100 μM PAA without external voltage. Mechanistic studies, including radical quenching, molecular probe validation, electron spin resonance (ESR) experiments, and density functional theory (DFT) calculations, revealed that SMX degradation was driven by reactive species generated via biogenic electron-mediated O3 C(O)OO• contributing 68.1 %, •OH of 18.4 %, and CH3 C(O)O• of 9.4 %, where initial formation of •OH and CH3 C(O)O• rapidly reacts with PAA to produce CH3 C(O)OO•. The presence of common water constituents such as anions (e.g., Cl− , NO3 − , and H2 PO4 − ) and humic acid (HA) significantly hinders SMX removal via the BES-PAA technique, whereas CO3 2− and HCO3 − ions have a comparatively minor impact. Additionally, the study investigated the removal of various pharmaceuticals present in secondary treated municipal wastewater, attributing differences in removal efficiency to the selective action of CH3 C(O)OO•. This research demonstrates a novel PAA activation method that is ecologically benign, inexpensive, and capable of overcoming catalyst deactivation and secondary pollution issues.
中文翻译:

使用生物电子通过电极激活过乙酸:一种消除药物的新型无能源和无催化剂工艺
过乙酸(PAA)作为废水处理的替代氧化剂受到越来越多的关注。然而,现有的PAA活化过程通常需要外部能量输入(例如,电和UV介导的活化)或催化剂(例如,Co2+),这不可避免地增加了处理成本或引入了需要额外去除的潜在新污染物。在这项工作中,我们在两室生物电化学系统 (BES) 中开发了一种无催化剂、自我维持的生物电化学方法,其中阴极电极使用阳极外放电极(例如地杆菌)降解产生的可再生生物电子原位激活 PAA阳极废水中的可生物降解有机物(例如乙酸)。这种创新的 BES-PAA 技术使用 100 μM PAA,无需外部电压,在 pH 2(阳离子交换膜)和 pH 6(双极膜)下,两小时内即可实现 98% 和 81% 的 2 µM 磺胺甲恶唑 (SMX) 去除率。包括自由基猝灭、分子探针验证、电子自旋共振 (ESR) 实验和密度泛函理论 (DFT) 计算在内的机理研究表明,SMX 降解是由生物电子介导的 PAA 与 CH3C 的 OO 裂解产生的活性物质驱动的。 (O)OO• 占 68.1 %,•OH 占 18.4 %,CH3C(O)O• 占 9.4 %,其中最初形成的•OH 和 CH3C(O)O• 快速与 PAA 反应生成 CH3C(O)OO •。常见水成分的存在,例如阴离子(例如,Cl−、NO3− 和 H2PO4−)和腐殖酸 (HA) 会显着阻碍通过 BES-PAA 技术去除 SMX,而 CO32− 和 HCO3− 离子则相对较小影响。 此外,该研究还调查了二级处理城市废水中存在的各种药物的去除情况,将去除效率的差异归因于 CH3C(O)OO• 的选择性作用。这项研究展示了一种新型的PAA活化方法,该方法对生态无害、成本低廉,并且能够克服催化剂失活和二次污染问题。
更新日期:2024-07-08
中文翻译:

使用生物电子通过电极激活过乙酸:一种消除药物的新型无能源和无催化剂工艺
过乙酸(PAA)作为废水处理的替代氧化剂受到越来越多的关注。然而,现有的PAA活化过程通常需要外部能量输入(例如,电和UV介导的活化)或催化剂(例如,Co2+),这不可避免地增加了处理成本或引入了需要额外去除的潜在新污染物。在这项工作中,我们在两室生物电化学系统 (BES) 中开发了一种无催化剂、自我维持的生物电化学方法,其中阴极电极使用阳极外放电极(例如地杆菌)降解产生的可再生生物电子原位激活 PAA阳极废水中的可生物降解有机物(例如乙酸)。这种创新的 BES-PAA 技术使用 100 μM PAA,无需外部电压,在 pH 2(阳离子交换膜)和 pH 6(双极膜)下,两小时内即可实现 98% 和 81% 的 2 µM 磺胺甲恶唑 (SMX) 去除率。包括自由基猝灭、分子探针验证、电子自旋共振 (ESR) 实验和密度泛函理论 (DFT) 计算在内的机理研究表明,SMX 降解是由生物电子介导的 PAA 与 CH3C 的 OO 裂解产生的活性物质驱动的。 (O)OO• 占 68.1 %,•OH 占 18.4 %,CH3C(O)O• 占 9.4 %,其中最初形成的•OH 和 CH3C(O)O• 快速与 PAA 反应生成 CH3C(O)OO •。常见水成分的存在,例如阴离子(例如,Cl−、NO3− 和 H2PO4−)和腐殖酸 (HA) 会显着阻碍通过 BES-PAA 技术去除 SMX,而 CO32− 和 HCO3− 离子则相对较小影响。 此外,该研究还调查了二级处理城市废水中存在的各种药物的去除情况,将去除效率的差异归因于 CH3C(O)OO• 的选择性作用。这项研究展示了一种新型的PAA活化方法,该方法对生态无害、成本低廉,并且能够克服催化剂失活和二次污染问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号