当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphonate Biochemistry
Chemical Reviews ( IF 51.4 ) Pub Date : 2016-10-27 00:00:00 , DOI: 10.1021/acs.chemrev.6b00536 Geoff P. Horsman 1 , David L. Zechel 2
Chemical Reviews ( IF 51.4 ) Pub Date : 2016-10-27 00:00:00 , DOI: 10.1021/acs.chemrev.6b00536 Geoff P. Horsman 1 , David L. Zechel 2
Affiliation
|
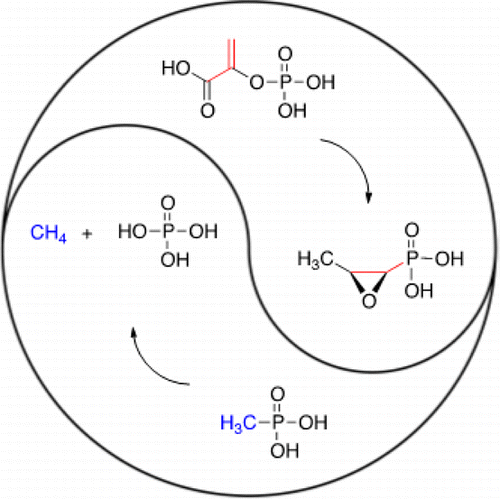
Organophosphonic acids are unique as natural products in terms of stability and mimicry. The C–P bond that defines these compounds resists hydrolytic cleavage, while the phosphonyl group is a versatile mimic of transition-states, intermediates, and primary metabolites. This versatility may explain why a variety of organisms have extensively explored the use organophosphonic acids as bioactive secondary metabolites. Several of these compounds, such as fosfomycin and bialaphos, figure prominently in human health and agriculture. The enzyme reactions that create these molecules are an interesting mix of chemistry that has been adopted from primary metabolism as well as those with no chemical precedent. Additionally, the phosphonate moiety represents a source of inorganic phosphate to microorganisms that live in environments that lack this nutrient; thus, unusual enzyme reactions have also evolved to cleave the C–P bond. This review is a comprehensive summary of the occurrence and function of organophosphonic acids natural products along with the mechanisms of the enzymes that synthesize and catabolize these molecules.
中文翻译:

膦酸酯生物化学
就稳定性和模仿性而言,有机膦酸作为天然产物是独特的。定义这些化合物的C–P键可抵抗水解裂解,而膦酰基则是过渡态,中间体和主要代谢物的通用模拟物。这种多功能性可以解释为什么许多生物广泛探索了使用有机膦酸作为生物活性次生代谢物的用途。这些化合物中的几种,例如磷霉素和双丙氨磷,在人类健康和农业中占有重要地位。产生这些分子的酶反应是化学的有趣混合,已被初级代谢以及没有化学先例的化学采用。另外,膦酸酯部分代表了生活在缺乏这种营养的环境中的微生物的无机磷酸盐的来源。因此,不寻常的酶反应也已经演化为裂解C-P键。这篇综述是有机膦酸天然产物的发生和功能的综合总结,以及合成和分解这些分子的酶的机理。
更新日期:2016-10-27
中文翻译:

膦酸酯生物化学
就稳定性和模仿性而言,有机膦酸作为天然产物是独特的。定义这些化合物的C–P键可抵抗水解裂解,而膦酰基则是过渡态,中间体和主要代谢物的通用模拟物。这种多功能性可以解释为什么许多生物广泛探索了使用有机膦酸作为生物活性次生代谢物的用途。这些化合物中的几种,例如磷霉素和双丙氨磷,在人类健康和农业中占有重要地位。产生这些分子的酶反应是化学的有趣混合,已被初级代谢以及没有化学先例的化学采用。另外,膦酸酯部分代表了生活在缺乏这种营养的环境中的微生物的无机磷酸盐的来源。因此,不寻常的酶反应也已经演化为裂解C-P键。这篇综述是有机膦酸天然产物的发生和功能的综合总结,以及合成和分解这些分子的酶的机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号