当前位置:
X-MOL 学术
›
Small Struct.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gadolinium-Manganese-Based Nanoplatform Reverses Radiotherapy Resistant Factors for Radiotherapy Sensitization and Computed Tomography/Magnetic Resonance Dual-Modal Imaging
Small Structures ( IF 13.9 ) Pub Date : 2024-07-10 , DOI: 10.1002/sstr.202400033 Yingwen Li 1, 2 , Panhong Niu 3 , Zhenzhong Han 3 , Xueqian Wang 2 , Duanmin Gao 3 , Yunjian Xu 1 , Qingbin He 1 , Jianfeng Qiu 1 , Yinglun Sun 1, 2
Small Structures ( IF 13.9 ) Pub Date : 2024-07-10 , DOI: 10.1002/sstr.202400033 Yingwen Li 1, 2 , Panhong Niu 3 , Zhenzhong Han 3 , Xueqian Wang 2 , Duanmin Gao 3 , Yunjian Xu 1 , Qingbin He 1 , Jianfeng Qiu 1 , Yinglun Sun 1, 2
Affiliation
|
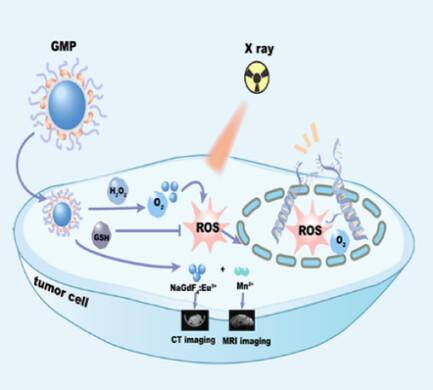
Insufficient reactive oxygen species originating from hypoxia and high glutathione (GSH) in the tumor microenvironment (TME) is an important reason for radiotherapy (RT) resistance. Currently, radiosensitizers that remodel TME are widely investigated to enhance RT. However, developing an effective nano-radiosensitization system that removes radiotherapy-resistant factors from TME to boost RT effect while visualizing tumor imaging to aid therapy remains a challenge. Herein, MnO2 nanosheets are grown on the surface of ultrasmall Eu-doped NaGdF4 (NaGdF4:Eu3+) nanoparticles and modified by biocompatible DSPE-PEG2000 to prepare NaGdF4:Eu3+@MnO2@PEG nanoparticles (denoted as GMP NPs) as a radiosensitizer, which not only can reverse the TME by degrading H2O2 to produce oxygen and consuming high GSH but also achieve computed tomography (CT)and magnetic resonance (MR) imaging. When GMP NPs synergize with X-ray, a better antitumor effect is achieved in both HeLa cells and tumor-bearing mice, compared with X-ray alone. In addition, both paramagnetic Mn2+ ionsproduced by decomposing MnO2 in TME and NaGdF4:Eu3+ nanoparticles enhance T1-weighted MR imaging. NaGdF4:Eu3+ nanoparticles containing high atomic number of Gd/Eu effectively attenuate X-ray to enhance CT imaging. The work provides new insights for developing an efficient RT sensitization platform integrating antitumor therapeutic effect as well as CT/MR dual-modal imaging.
中文翻译:

钆锰基纳米平台可逆转放疗耐药因素,实现放疗增敏和计算机断层扫描/磁共振双模态成像
肿瘤微环境(TME)中缺氧和高谷胱甘肽(GSH)引起的活性氧不足是放疗(RT)抵抗的重要原因。目前,重塑 TME 的放射增敏剂被广泛研究以增强 RT。然而,开发一种有效的纳米放射增敏系统,从 TME 中去除放射治疗抵抗因子以增强放疗效果,同时可视化肿瘤成像以辅助治疗仍然是一个挑战。本文中,MnO 2纳米片生长在超小的Eu掺杂NaGdF 4 (NaGdF 4 :Eu 3+ )纳米粒子的表面上,并通过生物相容性DSPE-PEG 2000进行修饰以制备NaGdF 4 :Eu 3+ @MnO 2 @PEG纳米粒子(表示为GMP NPs)作为放射增敏剂,不仅可以通过降解H 2 O 2产生氧气并消耗高GSH来逆转TME,而且还可以实现计算机断层扫描(CT)和磁共振(MR)成像。当 GMP NPs 与 X 射线协同作用时,与单独使用 X 射线相比,在 HeLa 细胞和荷瘤小鼠中都取得了更好的抗肿瘤效果。此外,通过在TME中分解MnO 2产生的顺磁Mn 2+离子和NaGdF 4 :Eu 3+纳米颗粒都增强了T 1加权MR成像。 NaGdF 4 :Eu 3+纳米粒子含有高原子序数的Gd/Eu,可有效衰减X射线以增强CT成像。该工作为开发整合抗肿瘤治疗效果以及CT/MR双模态成像的高效RT敏化平台提供了新的见解。
更新日期:2024-07-10
中文翻译:

钆锰基纳米平台可逆转放疗耐药因素,实现放疗增敏和计算机断层扫描/磁共振双模态成像
肿瘤微环境(TME)中缺氧和高谷胱甘肽(GSH)引起的活性氧不足是放疗(RT)抵抗的重要原因。目前,重塑 TME 的放射增敏剂被广泛研究以增强 RT。然而,开发一种有效的纳米放射增敏系统,从 TME 中去除放射治疗抵抗因子以增强放疗效果,同时可视化肿瘤成像以辅助治疗仍然是一个挑战。本文中,MnO 2纳米片生长在超小的Eu掺杂NaGdF 4 (NaGdF 4 :Eu 3+ )纳米粒子的表面上,并通过生物相容性DSPE-PEG 2000进行修饰以制备NaGdF 4 :Eu 3+ @MnO 2 @PEG纳米粒子(表示为GMP NPs)作为放射增敏剂,不仅可以通过降解H 2 O 2产生氧气并消耗高GSH来逆转TME,而且还可以实现计算机断层扫描(CT)和磁共振(MR)成像。当 GMP NPs 与 X 射线协同作用时,与单独使用 X 射线相比,在 HeLa 细胞和荷瘤小鼠中都取得了更好的抗肿瘤效果。此外,通过在TME中分解MnO 2产生的顺磁Mn 2+离子和NaGdF 4 :Eu 3+纳米颗粒都增强了T 1加权MR成像。 NaGdF 4 :Eu 3+纳米粒子含有高原子序数的Gd/Eu,可有效衰减X射线以增强CT成像。该工作为开发整合抗肿瘤治疗效果以及CT/MR双模态成像的高效RT敏化平台提供了新的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号