当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of Baloxavir Marboxil Using Diastereoselective Cyclization and Photoredox Decarboxylation of l-Serine
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-07-10 , DOI: 10.1021/acs.joc.4c00799 Kazuya Okamoto 1 , Tatsuhiko Ueno 2 , Yoshio Hato 2 , Yasunori Kawaguchi 3 , Toshikazu Hakogi 1 , Shohei Majima 1 , Takafumi Ohara 3 , Motoyuki Hagihara 3 , Norihiko Tanimoto 3 , Takayuki Tsuritani 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-07-10 , DOI: 10.1021/acs.joc.4c00799 Kazuya Okamoto 1 , Tatsuhiko Ueno 2 , Yoshio Hato 2 , Yasunori Kawaguchi 3 , Toshikazu Hakogi 1 , Shohei Majima 1 , Takafumi Ohara 3 , Motoyuki Hagihara 3 , Norihiko Tanimoto 3 , Takayuki Tsuritani 3
Affiliation
|
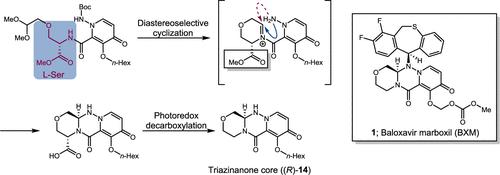
Baloxavir marboxil (1; BXM) is a potent drug used for treating influenza infections. The current synthetic route to BXM (1) is based on optical resolution; however, this method results in the loss of nearly 50% of the material. This study aimed to describe an efficient and simpler method for the synthesis of BXM. We achieved a stereoselective synthesis of BXM (1). The tricyclic triazinanone core possessing a chiral center was prepared via diastereoselective cyclization utilizing the readily available amino acid l-serine. The carboxyl moiety derived from l-serine was removed via photoredox decarboxylation under mild conditions to furnish the chiral tricyclic triazinanone core ((R)-14). The synthetic route demonstrated herein provides an efficient and atomically economical method for preparing this potent anti-influenza agent.
中文翻译:

使用 L-丝氨酸的非对映选择性环化和光氧化还原脱羧化立体选择性合成 Baloxavir Marboxil
Baloxavir marboxil (1;BXM) 是一种用于治疗流感感染的强效药物。目前通往 BXM (1) 的合成路线基于光学分辨率;然而,这种方法会导致近 50% 的材料损失。本研究旨在描述一种高效、简单的 BXM 合成方法。我们实现了 BXM 的立体选择性合成 (1)。利用现成的氨基酸 l-丝氨酸,通过非对映选择性环化制备具有手性中心的三环三嗪醌核心。在温和条件下通过光氧化还原脱羧去除源自 l-丝氨酸的羧基部分,以提供手性三环三嗪腺醌核心 ((R)-14)。本文演示的合成路线为制备这种有效的抗流感药物提供了一种有效且原子经济的方法。
更新日期:2024-07-10
中文翻译:

使用 L-丝氨酸的非对映选择性环化和光氧化还原脱羧化立体选择性合成 Baloxavir Marboxil
Baloxavir marboxil (1;BXM) 是一种用于治疗流感感染的强效药物。目前通往 BXM (1) 的合成路线基于光学分辨率;然而,这种方法会导致近 50% 的材料损失。本研究旨在描述一种高效、简单的 BXM 合成方法。我们实现了 BXM 的立体选择性合成 (1)。利用现成的氨基酸 l-丝氨酸,通过非对映选择性环化制备具有手性中心的三环三嗪醌核心。在温和条件下通过光氧化还原脱羧去除源自 l-丝氨酸的羧基部分,以提供手性三环三嗪腺醌核心 ((R)-14)。本文演示的合成路线为制备这种有效的抗流感药物提供了一种有效且原子经济的方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号