当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fixing the Achilles Heel of Pfizer’s Paxlovid for COVID-19 Treatment
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-07-05 , DOI: 10.1021/acs.jmedchem.4c01342 Lennart Brewitz 1 , Christopher J Schofield 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-07-05 , DOI: 10.1021/acs.jmedchem.4c01342 Lennart Brewitz 1 , Christopher J Schofield 1
Affiliation
|
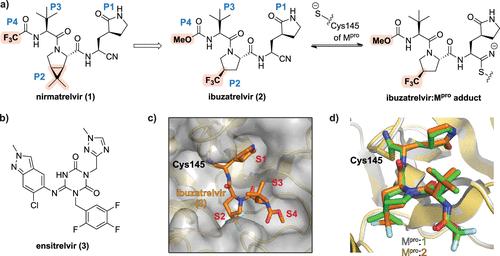
Nirmatrelvir (PF-07321332), a first-in-class inhibitor of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) main protease (Mpro), was developed by Pfizer under intense pressure during the pandemic to treat COVID-19. A weakness of nirmatrelvir is its limited metabolic stability, which led to the development of a combination therapy (paxlovid), involving coadministration of nirmatrelvir with the cytochrome P450 inhibitor ritonavir. However, limitations in tolerability of the ritonavir component reduce the scope of paxlovid. In response to these limitations, researchers at Pfizer have now developed the second-generation Mpro inhibitor PF-07817883 (ibuzatrelvir). Structurally related to nirmatrelvir, including with the presence of a trifluoromethyl group, albeit located differently, ibuzatrelvir manifests enhanced oral bioavailability, so it does not require coadministration with ritonavir. The development of ibuzatrelvir is an important milestone, because it is expected to enhance the treatment of COVID-19 without the drawbacks associated with ritonavir. Given the success of paxlovid in treating COVID-19, it is likely that ibuzatrelvir will be granted approval as an improved drug for treatment of COVID-19 infections, so complementing vaccination efforts and improving pandemic preparedness. The development of nirmatrelvir and ibuzatrelvir dramatically highlights the power of appropriately resourced modern medicinal chemistry to very rapidly enable the development of breakthrough medicines. Consideration of how analogous approaches can be used to develop similarly breakthrough medicines for infectious diseases such as tuberculosis and malaria is worthwhile.
中文翻译:

修复辉瑞 Paxlovid 治疗 COVID-19 的致命弱点
Nirmatrelvir(PF-07321332)是严重急性呼吸综合征冠状病毒2(SARS-CoV-2)主要蛋白酶(M pro )的一流抑制剂,由辉瑞公司在大流行期间的巨大压力下开发,用于治疗新冠肺炎-19。 nirmatrelvir 的一个弱点是其有限的代谢稳定性,这导致了联合疗法(paxlovid)的开发,包括 nirmatrelvir 与细胞色素 P450 抑制剂利托那韦的共同给药。然而,利托那韦成分的耐受性限制缩小了帕克洛维德的范围。针对这些限制,辉瑞的研究人员现已开发出第二代 M pro抑制剂 PF-07817883(ibuzatrelvir)。伊布扎韦在结构上与尼马瑞韦相关,包括存在三氟甲基,尽管位置不同,但伊布扎韦表现出增强的口服生物利用度,因此不需要与利托那韦共同给药。 ibuzatrelvir 的开发是一个重要的里程碑,因为它有望增强 COVID-19 的治疗效果,而不会出现与利托那韦相关的缺点。鉴于 Paxlovid 在治疗 COVID-19 方面取得的成功,ibuzatrelvir 很可能会被批准作为治疗 COVID-19 感染的改进药物,从而补充疫苗接种工作并改善大流行的防范。 nirmatrelvir 和 ibuzatrelvir 的开发极大地凸显了现代药物化学资源充足的力量,能够非常迅速地实现突破性药物的开发。值得考虑如何使用类似的方法来开发针对结核病和疟疾等传染病的类似突破性药物。
更新日期:2024-07-05
中文翻译:

修复辉瑞 Paxlovid 治疗 COVID-19 的致命弱点
Nirmatrelvir(PF-07321332)是严重急性呼吸综合征冠状病毒2(SARS-CoV-2)主要蛋白酶(M pro )的一流抑制剂,由辉瑞公司在大流行期间的巨大压力下开发,用于治疗新冠肺炎-19。 nirmatrelvir 的一个弱点是其有限的代谢稳定性,这导致了联合疗法(paxlovid)的开发,包括 nirmatrelvir 与细胞色素 P450 抑制剂利托那韦的共同给药。然而,利托那韦成分的耐受性限制缩小了帕克洛维德的范围。针对这些限制,辉瑞的研究人员现已开发出第二代 M pro抑制剂 PF-07817883(ibuzatrelvir)。伊布扎韦在结构上与尼马瑞韦相关,包括存在三氟甲基,尽管位置不同,但伊布扎韦表现出增强的口服生物利用度,因此不需要与利托那韦共同给药。 ibuzatrelvir 的开发是一个重要的里程碑,因为它有望增强 COVID-19 的治疗效果,而不会出现与利托那韦相关的缺点。鉴于 Paxlovid 在治疗 COVID-19 方面取得的成功,ibuzatrelvir 很可能会被批准作为治疗 COVID-19 感染的改进药物,从而补充疫苗接种工作并改善大流行的防范。 nirmatrelvir 和 ibuzatrelvir 的开发极大地凸显了现代药物化学资源充足的力量,能够非常迅速地实现突破性药物的开发。值得考虑如何使用类似的方法来开发针对结核病和疟疾等传染病的类似突破性药物。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号