当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study on the Condensation of 2-((4-Aminophenyl)sulfonyl)ethyl Hydrogen Sulfate with Alendronic Acid: Mechanism, Kinetics, and Reactor Modeling
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-07-08 , DOI: 10.1021/acs.iecr.4c02015 Xiao-Mei Ma 1 , Shen Li 2 , Hui-Long Wei 1 , Zheng-Hong Luo 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-07-08 , DOI: 10.1021/acs.iecr.4c02015 Xiao-Mei Ma 1 , Shen Li 2 , Hui-Long Wei 1 , Zheng-Hong Luo 1, 2
Affiliation
|
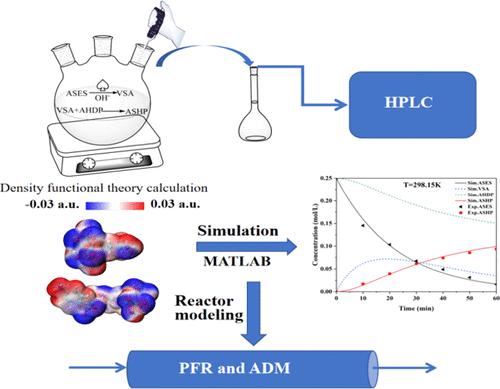
Self-dispersion dyes were prepared by introducing hydrophilic phosphate groups onto the surface of carbon black. 2-((4-Aminophenyl)sulfonyl)ethyl hydrogen sulfate and alendronic acid were used as the initial reactants to synthesize the key intermediate by a one-pot method, and the reaction network in this step was identified experimentally. Subsequently, density functional theory calculations were utilized to reveal the reaction mechanism and the energy barrier. Kinetic experiments were conducted at 288.15–303.15 K, and the experimental data were fitted by the nonlinear least-squares method to determine the rate constants at various temperatures. The activation energy and pre-exponential factor were further calculated by the Arrhenius relationship. Finally, a plug flow reactor model and an axial dispersion model were developed for the concept design of industrial continuous reactors, and detailed investigations on the effects of reactor parameters such as tube length, flow rate, wall temperature, and tube diameter on the conversion and yield were conducted.
中文翻译:

2-((4-氨基苯基)磺酰基)乙基硫酸氢盐与阿仑膦酸的缩合研究:机理、动力学和反应器模型
通过在炭黑表面引入亲水性磷酸基团制备自分散染料。以2-((4-氨基苯基)磺酰基)乙基硫酸氢盐和阿仑膦酸为初始反应物,通过一锅法合成了关键中间体,并通过实验确定了该步骤的反应网络。随后,利用密度泛函理论计算揭示了反应机理和能垒。动力学实验在288.15~303.15 K下进行,通过非线性最小二乘法对实验数据进行拟合,确定不同温度下的速率常数。通过阿伦尼乌斯关系进一步计算活化能和指前因子。最后,建立了活塞流反应器模型和轴向扩散模型,用于工业连续反应器的概念设计,并详细研究了反应器参数(如管长、流量、壁温和管径)对转化率和转化率的影响。进行了产量。
更新日期:2024-07-08
中文翻译:

2-((4-氨基苯基)磺酰基)乙基硫酸氢盐与阿仑膦酸的缩合研究:机理、动力学和反应器模型
通过在炭黑表面引入亲水性磷酸基团制备自分散染料。以2-((4-氨基苯基)磺酰基)乙基硫酸氢盐和阿仑膦酸为初始反应物,通过一锅法合成了关键中间体,并通过实验确定了该步骤的反应网络。随后,利用密度泛函理论计算揭示了反应机理和能垒。动力学实验在288.15~303.15 K下进行,通过非线性最小二乘法对实验数据进行拟合,确定不同温度下的速率常数。通过阿伦尼乌斯关系进一步计算活化能和指前因子。最后,建立了活塞流反应器模型和轴向扩散模型,用于工业连续反应器的概念设计,并详细研究了反应器参数(如管长、流量、壁温和管径)对转化率和转化率的影响。进行了产量。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号