当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Micro-nano H2 bubbles enhanced hydrodehalogenation of 3-chloro-4-fluoroaniline: Mass transfer and action mechanism
Chemosphere ( IF 8.1 ) Pub Date : 2024-07-08 , DOI: 10.1016/j.chemosphere.2024.142816 Weilai Wang 1 , Xinting Guo 1 , Zekun Liu 1 , Shuang Dong 1 , Haijin Liu 1 , Yuandong Wu 2 , Zhiguo Cao 1
Chemosphere ( IF 8.1 ) Pub Date : 2024-07-08 , DOI: 10.1016/j.chemosphere.2024.142816 Weilai Wang 1 , Xinting Guo 1 , Zekun Liu 1 , Shuang Dong 1 , Haijin Liu 1 , Yuandong Wu 2 , Zhiguo Cao 1
Affiliation
|
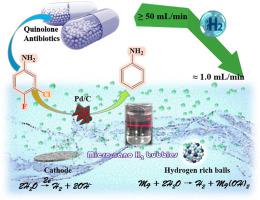
3-chloro-4-fluoraniline (FCA) is an important intermediate for the synthesis of antibiotics, herbicides and insecticides, and has significant environmental health hazards. Catalytic hydrogenation technology is widely used in pretreatment of halogenated organics due to its simple process and excellent performance. However, compared with the research of high activity hydrogenation catalyst, the research of efficient utilization of hydrogen source under mild conditions is not sufficient. In this work, micro-nano H bubbles are produced in situ by electrolytic water and active metal replacement, and their apparent properties are studied. The result show that the H bubbles have a size distribution in the range of 150–900 nm, which can rapidly reduce the REDOX potential of the water and maintain it in a hydrogen-rich state for a long time. Under the action of Pd/C catalyst, atomic hydrogen (H•) produced by dissociative adsorption can sequentially hydrogenate FCA to aniline. The H• utilization ratios of the above two hydrogen supply pathways reach 6.20% and 4.94% respectively, and H consumption is reduced by tens of times (≥50 → ≈1.0 mL/min). The research provides technical support for the efficient removal of halogenated refractory pollutants in water and the development of hydrogen economy.
中文翻译:

微纳米氢气气泡增强3-氯-4-氟苯胺加氢脱卤反应:传质及作用机制
3-氯-4-氟苯胺(FCA)是合成抗生素、除草剂和杀虫剂的重要中间体,具有显着的环境健康危害。催化加氢技术因其工艺简单、性能优异而广泛应用于卤代有机物的预处理。然而,与高活性加氢催化剂的研究相比,温和条件下氢源高效利用的研究还不够充分。本工作通过电解水和活性金属置换原位产生微纳米H气泡,并研究其表观性质。结果表明,H气泡的尺寸分布在150~900 nm范围内,能够迅速降低水的氧化还原电位,并长期保持水的富氢状态。在Pd/C催化剂的作用下,解离吸附产生的原子氢(H•)可以将FCA依次氢化成苯胺。上述两种供氢途径的H•利用率分别达到6.20%和4.94%,H2消耗降低数十倍(≥50→≈1.0 mL/min)。该研究为高效去除水中卤代难降解污染物、发展氢经济提供技术支撑。
更新日期:2024-07-08
中文翻译:

微纳米氢气气泡增强3-氯-4-氟苯胺加氢脱卤反应:传质及作用机制
3-氯-4-氟苯胺(FCA)是合成抗生素、除草剂和杀虫剂的重要中间体,具有显着的环境健康危害。催化加氢技术因其工艺简单、性能优异而广泛应用于卤代有机物的预处理。然而,与高活性加氢催化剂的研究相比,温和条件下氢源高效利用的研究还不够充分。本工作通过电解水和活性金属置换原位产生微纳米H气泡,并研究其表观性质。结果表明,H气泡的尺寸分布在150~900 nm范围内,能够迅速降低水的氧化还原电位,并长期保持水的富氢状态。在Pd/C催化剂的作用下,解离吸附产生的原子氢(H•)可以将FCA依次氢化成苯胺。上述两种供氢途径的H•利用率分别达到6.20%和4.94%,H2消耗降低数十倍(≥50→≈1.0 mL/min)。该研究为高效去除水中卤代难降解污染物、发展氢经济提供技术支撑。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号