当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anionic polymerization of phenyl-substituted isoprene derivatives: polymerization behaviour and cyclization-enabled fluorescence
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-07-10 , DOI: 10.1039/d4py00601a Moritz Rauschenbach 1 , Laura Stein 1 , Gregor M. Linden 1 , Ramona Barent 1, 2 , Katja Heinze 1 , Holger Frey 1
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-07-10 , DOI: 10.1039/d4py00601a Moritz Rauschenbach 1 , Laura Stein 1 , Gregor M. Linden 1 , Ramona Barent 1, 2 , Katja Heinze 1 , Holger Frey 1
Affiliation
|
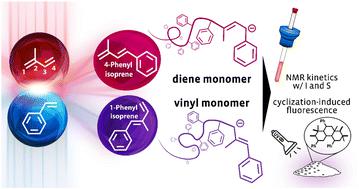
1,3-Dienes are important monomers for living anionic polymerization. However, phenyl-substituted diene monomer structures have been rarely investigated. Based on DFT calculations and 13C NMR spectroscopy, a discrepancy in the reactivity of the two monomers 1-phenyl isoprene (1PhI) and 4-phenyl isoprene (4PhI) in anionic polymerization is expected. Starting from a Wittig reaction including an optimized extraction procedure, disubstituted 1,3-dienes were obtained that resulted in polymers with different degrees of 1,3-incorporation. The polymers have been characterized by 1H NMR spectroscopy and using different SEC conditions. Molecular weights of up to 48.8 kg mol−1 with narrow dispersities (Đ ≤ 1.13) were achieved. The addition of the modifier THF led to an initial increase of vinyl units as well as a loss of control over the polymerization of 4PhI. Increasing the THF concentration further resulted in a rather unusual decrease of the vinyl units and ended with more than 80% 1,4-units in pure THF. Copolymerizations with styrene (S) and isoprene (I), respectively, were tracked via in situ 1H NMR kinetics. The observed ideally random copolymerizations of I and 1PhI as well as the gradient copolymers with S were further investigated via the synthesis of copolymers with a targeted Mn of 40 kg mol−1. In a subsequent reaction step, the homopolymers were cyclized using trifluoromethyl sulfonic acid inducing fluorescence properties. The different microstructures and substitution patterns of the original polymers differ in both emission maxima and quantum yields.
中文翻译:

苯基取代异戊二烯衍生物的阴离子聚合:聚合行为和环化荧光
1,3-二烯是活性阴离子聚合的重要单体。然而,苯基取代的二烯单体结构很少被研究。基于DFT计算和 13 C NMR光谱,预计阴离子聚合中两种单体1-苯基异戊二烯(1PhI)和4-苯基异戊二烯(4PhI)的反应性存在差异。从维蒂希反应(包括优化的萃取程序)开始,获得了双取代的 1,3-二烯,从而产生了具有不同 1,3- 结合程度的聚合物。该聚合物已通过 1 H NMR 光谱并使用不同的SEC 条件进行了表征。分子量高达 48.8 kg mol −1 ,且分散度较窄 (Đ ≤ 1.13)。改性剂 THF 的添加导致乙烯基单元的初始增加以及对 4PhI 聚合的控制丧失。增加 THF 浓度进一步导致乙烯基单元的相当不寻常的减少,最终纯 THF 中含有超过 80% 的 1,4-单元。通过原位 1 H NMR 动力学分别追踪与苯乙烯(S) 和异戊二烯(I) 的共聚。通过合成目标 M n 为 40 kg mol −1 的共聚物,进一步研究了观察到的 I 和 1PhI 的理想无规共聚以及与 S 的梯度共聚物。在随后的反应步骤中,使用三氟甲基磺酸诱导荧光特性将均聚物环化。原始聚合物的不同微观结构和取代模式在发射最大值和量子产率方面都不同。
更新日期:2024-07-10
中文翻译:

苯基取代异戊二烯衍生物的阴离子聚合:聚合行为和环化荧光
1,3-二烯是活性阴离子聚合的重要单体。然而,苯基取代的二烯单体结构很少被研究。基于DFT计算和 13 C NMR光谱,预计阴离子聚合中两种单体1-苯基异戊二烯(1PhI)和4-苯基异戊二烯(4PhI)的反应性存在差异。从维蒂希反应(包括优化的萃取程序)开始,获得了双取代的 1,3-二烯,从而产生了具有不同 1,3- 结合程度的聚合物。该聚合物已通过 1 H NMR 光谱并使用不同的SEC 条件进行了表征。分子量高达 48.8 kg mol −1 ,且分散度较窄 (Đ ≤ 1.13)。改性剂 THF 的添加导致乙烯基单元的初始增加以及对 4PhI 聚合的控制丧失。增加 THF 浓度进一步导致乙烯基单元的相当不寻常的减少,最终纯 THF 中含有超过 80% 的 1,4-单元。通过原位 1 H NMR 动力学分别追踪与苯乙烯(S) 和异戊二烯(I) 的共聚。通过合成目标 M n 为 40 kg mol −1 的共聚物,进一步研究了观察到的 I 和 1PhI 的理想无规共聚以及与 S 的梯度共聚物。在随后的反应步骤中,使用三氟甲基磺酸诱导荧光特性将均聚物环化。原始聚合物的不同微观结构和取代模式在发射最大值和量子产率方面都不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号