当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoredox-enabled ring-opening of cyclobutanes via the formation of a carbon radical
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-10 , DOI: 10.1039/d4qo00996g Chunhang Zhao 1 , Wenjing Ma 1 , Kairui Liu 1 , Ruoyang Xu 1 , Xiuya Ma 1 , Yan Zhang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-10 , DOI: 10.1039/d4qo00996g Chunhang Zhao 1 , Wenjing Ma 1 , Kairui Liu 1 , Ruoyang Xu 1 , Xiuya Ma 1 , Yan Zhang 1
Affiliation
|
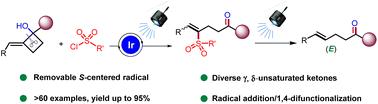
The high strain energy of cyclobutanes has been utilized for selective ring-opening, enabling the synthesis of valuable linear aliphatic compounds. Although significant progress has been observed recently, most of the approaches appear to be limited to two major manifolds, namely, transition metal-catalyzed carbon–carbon (C–C) oxidative addition or cyclobutyloxy radical-initiated ring opening. In sharp contrast, we describe photoinduced C–C activation using a cyclobutylcarbinyl radical to access γ,δ-unsaturated ketones obtained from cyclobutyl tertiary alcohols and sulfonyl chlorides through a strain release strategy associated with the formation of a stabilized radical. Furthermore, the ketocarbonyl group also enables diverse post-synthetic modifications. Overall, our approach is characterized by broad functional group tolerance, ample substrate scope, and scalability. Our findings represent an alternative method to the aforementioned methods for remote 1,4-difunctionalizations, accompanied by a synthetically useful C–C double bond persisting in the obtained products.
中文翻译:

通过形成碳自由基实现环丁烷的光氧化还原开环
环丁烷的高应变能已被用于选择性开环,从而能够合成有价值的直链脂肪族化合物。尽管最近已经观察到重大进展,但大多数方法似乎仅限于两个主要方面,即过渡金属催化的碳-碳(C-C)氧化加成或环丁氧基自由基引发的开环。与此形成鲜明对比的是,我们描述了光诱导的 C-C 活化,使用环丁基羰基自由基通过与稳定自由基形成相关的应变释放策略来获取从环丁基叔醇和磺酰氯获得的 γ,δ-不饱和酮。此外,酮羰基还可以进行多种合成后修饰。总体而言,我们的方法的特点是广泛的官能团耐受性、充足的底物范围和可扩展性。我们的研究结果代表了上述远程 1,4-双官能化方法的替代方法,并伴随着所获得的产物中持续存在的合成有用的 C-C 双键。
更新日期:2024-07-15
中文翻译:

通过形成碳自由基实现环丁烷的光氧化还原开环
环丁烷的高应变能已被用于选择性开环,从而能够合成有价值的直链脂肪族化合物。尽管最近已经观察到重大进展,但大多数方法似乎仅限于两个主要方面,即过渡金属催化的碳-碳(C-C)氧化加成或环丁氧基自由基引发的开环。与此形成鲜明对比的是,我们描述了光诱导的 C-C 活化,使用环丁基羰基自由基通过与稳定自由基形成相关的应变释放策略来获取从环丁基叔醇和磺酰氯获得的 γ,δ-不饱和酮。此外,酮羰基还可以进行多种合成后修饰。总体而言,我们的方法的特点是广泛的官能团耐受性、充足的底物范围和可扩展性。我们的研究结果代表了上述远程 1,4-双官能化方法的替代方法,并伴随着所获得的产物中持续存在的合成有用的 C-C 双键。












































 京公网安备 11010802027423号
京公网安备 11010802027423号