当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Total synthesis of (–)-deglycocadambine
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-10 , DOI: 10.1039/d4qo01122h Fang-Xin Wang 1 , Ying-Tao Chen 1 , Hui Liu 1 , Heng-Shan Wang 1 , Hong Liang 1 , Zhen-Feng Chen 1 , Yonggui Robin Chi 2, 3
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-10 , DOI: 10.1039/d4qo01122h Fang-Xin Wang 1 , Ying-Tao Chen 1 , Hui Liu 1 , Heng-Shan Wang 1 , Hong Liang 1 , Zhen-Feng Chen 1 , Yonggui Robin Chi 2, 3
Affiliation
|
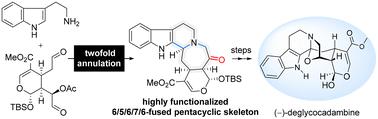
The first total synthesis of the monoterpene indole alkaloid (–)-deglycocadambine is achieved in 12 steps with (+)-genipin as the chiral starting material. The reported synthetic approach is characterized by an orchestrated cascade annulation between tryptamine and the highly functionalized dialdehyde precursor, rapidly introducing the unique 6/5/6/7/6-fused pentacyclic skeleton and the ketone functional group at C19 in a convergent manner. The successful implementation of transannular oxidative cyclization at C3 for bridged oxazolidine formation in the late-stage synthetic campaign ensured the final total synthesis of this molecule.
中文翻译:

(–)-去糖卡达宾的全合成
以 (+)-京尼平为手性起始原料,经过 12 个步骤实现了单萜吲哚生物碱 (-)-去糖卡达宾的首次全合成。报道的合成方法的特点是色胺和高度功能化的二醛前体之间精心策划的级联环化,以收敛的方式快速引入独特的6/5/6/7/6稠合五环骨架和C19处的酮官能团。在后期合成过程中,C3 处跨环氧化环化形成桥联恶唑烷的成功确保了该分子的最终全合成。
更新日期:2024-07-10
中文翻译:

(–)-去糖卡达宾的全合成
以 (+)-京尼平为手性起始原料,经过 12 个步骤实现了单萜吲哚生物碱 (-)-去糖卡达宾的首次全合成。报道的合成方法的特点是色胺和高度功能化的二醛前体之间精心策划的级联环化,以收敛的方式快速引入独特的6/5/6/7/6稠合五环骨架和C19处的酮官能团。在后期合成过程中,C3 处跨环氧化环化形成桥联恶唑烷的成功确保了该分子的最终全合成。












































 京公网安备 11010802027423号
京公网安备 11010802027423号