当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Safety Guided Process Development and Scale-Up of the Highly Energetic Compound 4-Methyl-1,2,5-oxadiazole-3-carboxylic Acid
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-05 , DOI: 10.1021/acs.oprd.4c00137 Qiang Yang 1 , Yu Lu 1 , Han Xia 1 , Vishaal Gopalakrishnan 1 , Scott A. Frank 1 , Yungang He 2 , Lixuan Liang 2 , Xin Zhang 2 , Ping Huang 2 , Chuanren Liu 2 , Jing Chen 2 , Qicheng Ma 2 , Sha Li 2 , Li Fan 2
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-07-05 , DOI: 10.1021/acs.oprd.4c00137 Qiang Yang 1 , Yu Lu 1 , Han Xia 1 , Vishaal Gopalakrishnan 1 , Scott A. Frank 1 , Yungang He 2 , Lixuan Liang 2 , Xin Zhang 2 , Ping Huang 2 , Chuanren Liu 2 , Jing Chen 2 , Qicheng Ma 2 , Sha Li 2 , Li Fan 2
Affiliation
|
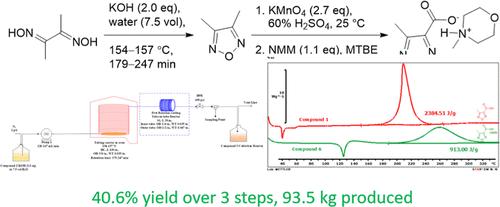
The synthesis of the highly energetic pharmaceutical intermediate 4-methyl-1,2,5-oxadiazole-3-carboxylic acid via cyclization of (2E,3E)-butane-2,3-dione dioxime followed by selective oxidation of the resulting 3,4-dimethyl-1,2,5-oxadiazole was developed to support the sample production for clinical trials. Rigorous process safety evaluations revealed many potential safety risks associated with the involved highly energetic compounds and the high exothermicity of the oxidation reaction. A continuous flow process was developed for the synthesis of 3,4-dimethyl-1,2,5-oxadiazole to mitigate the potential safety risks associated with the thermal instability of starting material (2E,3E)-butane-2,3-dione dioxime and product 3,4-dimethyl-1,2,5-oxadiazole. A much safer process for the highly exothermic oxidation of 3,4-dimethyl-1,2,5-oxadiazole involving portion-wise addition of potassium permanganate was developed to avoid accumulation of reactive chemicals. The desired product was isolated as N-methyl morpholine 4-methyl-1,2,5-oxadiazole-3-carboxylate to avoid handling of potentially explosive 4-methyl-1,2,5-oxadiazole-3-carboxylic acid in its solid form. The developed process was successfully scaled up to afford a total of 93.52 kg of N-methyl morpholine 4-methyl-1,2,5-oxadiazole-3-carboxylate to support the production of a drug substance.
中文翻译:

高能化合物 4-甲基-1,2,5-恶二唑-3-甲酸的安全引导工艺开发和放大
通过 (2E,3E)-丁烷-2,3-二酮二肟环化,然后选择性氧化所得 3,合成高能药物中间体 4-甲基-1,2,5-恶二唑-3-羧酸, 4-二甲基-1,2,5-恶二唑的开发是为了支持临床试验的样品生产。严格的过程安全评估揭示了许多与所涉及的高能化合物和氧化反应的高放热相关的潜在安全风险。开发了一种连续流动工艺来合成 3,4-二甲基-1,2,5-恶二唑,以减轻与起始原料 (2E,3E)-丁烷-2,3-二酮的热不稳定性相关的潜在安全风险二肟和产物3,4-二甲基-1,2,5-恶二唑。开发了一种更安全的 3,4-二甲基-1,2,5-恶二唑高放热氧化工艺,涉及分批添加高锰酸钾,以避免反应性化学物质的积累。所需产物被分离为 N-甲基吗啉 4-甲基-1,2,5-恶二唑-3-羧酸酯,以避免在其固体中处理潜在爆炸性的 4-甲基-1,2,5-恶二唑-3-羧酸形式。所开发的工艺已成功扩大规模,总共可生产 93.52 千克 N-甲基吗啉 4-甲基-1,2,5-恶二唑-3-甲酸,以支持原料药的生产。
更新日期:2024-07-05
中文翻译:

高能化合物 4-甲基-1,2,5-恶二唑-3-甲酸的安全引导工艺开发和放大
通过 (2E,3E)-丁烷-2,3-二酮二肟环化,然后选择性氧化所得 3,合成高能药物中间体 4-甲基-1,2,5-恶二唑-3-羧酸, 4-二甲基-1,2,5-恶二唑的开发是为了支持临床试验的样品生产。严格的过程安全评估揭示了许多与所涉及的高能化合物和氧化反应的高放热相关的潜在安全风险。开发了一种连续流动工艺来合成 3,4-二甲基-1,2,5-恶二唑,以减轻与起始原料 (2E,3E)-丁烷-2,3-二酮的热不稳定性相关的潜在安全风险二肟和产物3,4-二甲基-1,2,5-恶二唑。开发了一种更安全的 3,4-二甲基-1,2,5-恶二唑高放热氧化工艺,涉及分批添加高锰酸钾,以避免反应性化学物质的积累。所需产物被分离为 N-甲基吗啉 4-甲基-1,2,5-恶二唑-3-羧酸酯,以避免在其固体中处理潜在爆炸性的 4-甲基-1,2,5-恶二唑-3-羧酸形式。所开发的工艺已成功扩大规模,总共可生产 93.52 千克 N-甲基吗啉 4-甲基-1,2,5-恶二唑-3-甲酸,以支持原料药的生产。






























 京公网安备 11010802027423号
京公网安备 11010802027423号