Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-07-08 , DOI: 10.1016/j.stem.2024.06.004 Yue Liu 1 , Yung Su Kim 1 , Xufeng Xue 1 , Yuchuan Miao 2 , Norio Kobayashi 1 , Shiyu Sun 1 , Robin Zhexuan Yan 1 , Qiong Yang 3 , Olivier Pourquié 4 , Jianping Fu 5
|
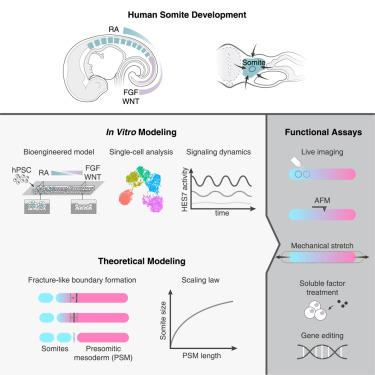
Emerging human pluripotent stem cell (hPSC)-based embryo models are useful for studying human embryogenesis. Particularly, there are hPSC-based somitogenesis models using free-floating culture that recapitulate somite formation. Somitogenesis in vivo involves intricately orchestrated biochemical and biomechanical events. However, none of the current somitogenesis models controls biochemical gradients or biomechanical signals in the culture, limiting their applicability to untangle complex biochemical-biomechanical interactions that drive somitogenesis. Herein, we develop a human somitogenesis model by confining hPSC-derived presomitic mesoderm (PSM) tissues in microfabricated trenches. Exogenous microfluidic morphogen gradients imposed on the PSM tissues cause axial patterning and trigger spontaneous rostral-to-caudal somite formation. A mechanical theory is developed to explain the size dependency between somites and the PSM. The microfluidic somitogenesis model is further exploited to reveal regulatory roles of cellular and tissue biomechanics in somite formation. This study presents a useful microengineered, hPSC-based model for understanding the biochemical and biomechanical events that guide somite formation.
中文翻译:

使用微流体的基于人类多能干细胞的体细胞发生模型
新兴的基于人类多能干细胞(hPSC)的胚胎模型可用于研究人类胚胎发生。特别是,有基于 hPSC 的体节发生模型,使用自由漂浮培养物来概括体节形成。体内体细胞发生涉及错综复杂的生物化学和生物力学事件。然而,目前的体细胞发生模型都无法控制培养物中的生化梯度或生物力学信号,这限制了它们在解开驱动体细胞发生的复杂生化-生物力学相互作用方面的适用性。在此,我们通过将 hPSC 衍生的前体中胚层 (PSM) 组织限制在微型沟槽中,开发了人类体细胞发生模型。施加在 PSM 组织上的外源微流体形态发生素梯度会导致轴向图案化并触发自发的头尾体节形成。发展了一种力学理论来解释体节和 PSM 之间的尺寸依赖性。微流体体节发生模型被进一步利用来揭示细胞和组织生物力学在体节形成中的调节作用。这项研究提出了一种有用的基于 hPSC 的微工程模型,用于了解指导体节形成的生化和生物力学事件。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号