当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni/photoredox-catalyzed coupling of aryl bromides and methylenecyclopropanes via selective distal bond cleavage
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-09 , DOI: 10.1039/d4qo01072h Ben Mao 1 , Min Shi 1, 2 , Yin Wei 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-09 , DOI: 10.1039/d4qo01072h Ben Mao 1 , Min Shi 1, 2 , Yin Wei 2
Affiliation
|
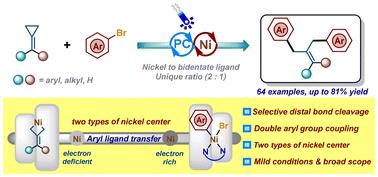
C(sp3) hybridized fragments have been widely explored for the formation of crucial Ni-alkyl intermediates in the field of nickel-catalyzed C(sp2)–C(sp3) coupling reactions. Traditional alkyl metal reagents and diverse C(sp3) radical precursors developed with the renascence of photocatalysis are effective coupling partners. However, though Ni-alkyl intermediates can be readily obtained to activate carbon–carbon bonds, cyclopropane derivatives have rarely been employed as partners to couple with aryl halides. Herein, we disclose a Ni/photoredox protocol for coupling methylenecyclopropanes with aryl bromides via selective distal bond cleavage. A range of 1,1-dibenzylethylene derivatives has been obtained in moderate-to-good yields under mild conditions with excellent functional group tolerance. Mechanistic studies demonstrate the unique ratio of the nickel catalyst to bidentate ligand (Ni/Ligand = 2/1) utilized in this reaction, which was derived from two distinct roles of nickel played in the oxidative addition step with MCP substrates and aryl bromides. This context presents an unusual catalytic mode in transition-metal-catalyzed transformations of methylenecyclopropanes.
中文翻译:

Ni/光氧化还原催化芳基溴和亚甲基环丙烷通过选择性远端键断裂偶联
C(sp 3 ) 杂化片段已被广泛探索用于镍催化 C(sp 2 )–C(sp 3 ) 偶联反应。传统的烷基金属试剂和随着光催化的复兴而开发的各种C(sp 3 )自由基前体是有效的偶联伙伴。然而,尽管可以很容易地获得镍烷基中间体来活化碳-碳键,但环丙烷衍生物很少被用作与芳基卤化物的偶联物。在此,我们公开了通过选择性远端键断裂将亚甲基环丙烷与芳基溴偶联的Ni/光氧化还原方案。在温和条件下以中等至良好的产率获得了一系列具有优异官能团耐受性的 1,1-二苄基乙烯衍生物。机理研究证明了该反应中使用的镍催化剂与二齿配体的独特比例(Ni/配体= 2/1),这是由于镍在MCP底物和芳基溴的氧化加成步骤中发挥的两种不同作用。本文提出了亚甲基环丙烷过渡金属催化转化中一种不寻常的催化模式。
更新日期:2024-07-09
中文翻译:

Ni/光氧化还原催化芳基溴和亚甲基环丙烷通过选择性远端键断裂偶联
C(sp 3 ) 杂化片段已被广泛探索用于镍催化 C(sp 2 )–C(sp 3 ) 偶联反应。传统的烷基金属试剂和随着光催化的复兴而开发的各种C(sp 3 )自由基前体是有效的偶联伙伴。然而,尽管可以很容易地获得镍烷基中间体来活化碳-碳键,但环丙烷衍生物很少被用作与芳基卤化物的偶联物。在此,我们公开了通过选择性远端键断裂将亚甲基环丙烷与芳基溴偶联的Ni/光氧化还原方案。在温和条件下以中等至良好的产率获得了一系列具有优异官能团耐受性的 1,1-二苄基乙烯衍生物。机理研究证明了该反应中使用的镍催化剂与二齿配体的独特比例(Ni/配体= 2/1),这是由于镍在MCP底物和芳基溴的氧化加成步骤中发挥的两种不同作用。本文提出了亚甲基环丙烷过渡金属催化转化中一种不寻常的催化模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号