当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral phosphoric acid-catalyzed enantioselective synthesis of biphenyl-bridged ε-sultams via the Friedel–Crafts reactions of cyclic N-sulfonylimines with indolizines
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-09 , DOI: 10.1039/d4qo01037j Qian Li 1 , Yuan-Yuan Xu 1 , Bi-Xi Feng 2 , Tao Wang 1 , You-Qing Wang 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-09 , DOI: 10.1039/d4qo01037j Qian Li 1 , Yuan-Yuan Xu 1 , Bi-Xi Feng 2 , Tao Wang 1 , You-Qing Wang 1
Affiliation
|
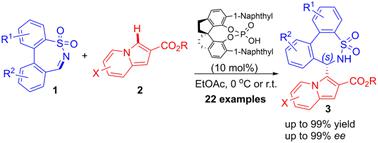
A highly enantioselective aza-Friedel–Crafts arylation of biphenyl-bridged seven-membered cyclic N-sulfonylimines with indolizines was developed, affording a wide range of chiral indolizine modified biphenyl-bridged ε-sultams in excellent yields (up to 99% yield) and enantioselectivities (up to 99% ee) by utilizing chiral phosphoric acid organocatalysis. Furthermore, scale-up reactions and diversified synthetic transformations of the desired ε-sultams without the loss of stereochemical purity substantiated their potential utility values.
中文翻译:

手性磷酸催化通过环状 N-磺酰亚胺与中氮茚的 Friedel-Crafts 反应对映选择性合成联苯桥联 ε-磺内酰胺
开发了联苯桥七元环状 N-磺酰亚胺与中氮茚的高度对映选择性氮杂-弗里德尔-克来福特芳基化反应,以优异的产率(高达 99% 产率)提供了多种手性中氮茚修饰的联苯桥 ε-磺内酰胺利用手性磷酸有机催化实现对映选择性(高达 99% ee)。此外,在不损失立体化学纯度的情况下,所需ε-磺内酰胺的放大反应和多样化合成转化证实了其潜在的实用价值。
更新日期:2024-07-09
中文翻译:

手性磷酸催化通过环状 N-磺酰亚胺与中氮茚的 Friedel-Crafts 反应对映选择性合成联苯桥联 ε-磺内酰胺
开发了联苯桥七元环状 N-磺酰亚胺与中氮茚的高度对映选择性氮杂-弗里德尔-克来福特芳基化反应,以优异的产率(高达 99% 产率)提供了多种手性中氮茚修饰的联苯桥 ε-磺内酰胺利用手性磷酸有机催化实现对映选择性(高达 99% ee)。此外,在不损失立体化学纯度的情况下,所需ε-磺内酰胺的放大反应和多样化合成转化证实了其潜在的实用价值。












































 京公网安备 11010802027423号
京公网安备 11010802027423号