Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and mutagenic risk of avanafil's potential genotoxic impurities
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-08 , DOI: 10.1039/d4ra02345e Yunkai Sun 1, 2 , Xiaoxia Wu 2, 3 , Pei Zuo 3 , Zhao Liu 3 , Xuepei Miao 1 , Jian Liu 1 , Hairuo Wen 4
RSC Advances ( IF 3.9 ) Pub Date : 2024-07-08 , DOI: 10.1039/d4ra02345e Yunkai Sun 1, 2 , Xiaoxia Wu 2, 3 , Pei Zuo 3 , Zhao Liu 3 , Xuepei Miao 1 , Jian Liu 1 , Hairuo Wen 4
Affiliation
|
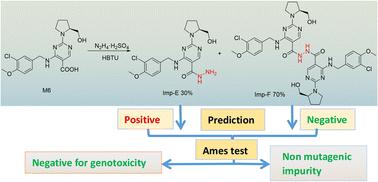
In the technical route for the synthesis of avanafil, 1-ethyl-(3-dimethylaminopropyl)carbamyldiimide hydrochloride (EDCI) and 1-hydroxybenzotriazole (HOBT) are used as reactive acid–amine binding agents. HOBT contains trace amounts of hydrazine residue, and there is a risk of introducing potentially mutagenic impurities with hydrazide-containing structures. The potentially genotoxic impurities E (Imp-E) and F (Imp-F) of avanafil with altering hydrazide-structure were synthesized by chemical method; subsequently, the impurities were evaluated and classified according to ICH M7 guidelines. Two complementary quantitative structure–activity relationship (QSAR) evaluation systems (Derek and Sarah) based on expert rules and statistics were used to preliminarily predict the genotoxicity of Imp-E and Imp-F, and the prediction result of E was suspected to be positive. In the Ames test of Imp-E and Imp-F, in the dose range of 62.5–1000 μg per plate, with or without the presence of metabolic activation system S9, the number of revertant colonies did not exceed 2 times the number of colonies in the solvent control group and did not show a dose–response relationship, and the test results were negative. Imp-E and Imp-F were determined to be negative for genotoxicity, which could be controlled as class 5 in ICH M7, that is, non mutagenic impurity.
中文翻译:

阿伐那非潜在基因毒性杂质的合成和致突变风险
在阿伐那非的合成技术路线中,采用1-乙基-(3-二甲氨基丙基)氨甲酰二亚胺盐酸盐(EDCI)和1-羟基苯并三唑(HOBT)作为反应性酸胺结合剂。 HOBT含有微量的肼残留物,并且存在引入具有含酰肼结构的潜在致突变杂质的风险。采用化学方法合成了改变酰肼结构的阿伐那非潜在遗传毒性杂质E( Imp-E )和F( Imp-F );随后,根据 ICH M7 指南对杂质进行评估和分类。采用两个基于专家规则和统计的互补定量构效关系(QSAR)评价系统(Derek和Sarah)初步预测Imp-E和Imp-F的遗传毒性,E的预测结果疑似阳性。 Imp-E和Imp-F的Ames试验中,在每板62.5~1000μg的剂量范围内,有或没有代谢活化系统S9存在时,回复菌落数不超过菌落数的2倍溶剂对照组未表现出剂量反应关系,试验结果呈阴性。 Imp-E和Imp-F经测定遗传毒性呈阴性,可控制为ICH M7中的5类,即非致突变杂质。
更新日期:2024-07-08
中文翻译:

阿伐那非潜在基因毒性杂质的合成和致突变风险
在阿伐那非的合成技术路线中,采用1-乙基-(3-二甲氨基丙基)氨甲酰二亚胺盐酸盐(EDCI)和1-羟基苯并三唑(HOBT)作为反应性酸胺结合剂。 HOBT含有微量的肼残留物,并且存在引入具有含酰肼结构的潜在致突变杂质的风险。采用化学方法合成了改变酰肼结构的阿伐那非潜在遗传毒性杂质E( Imp-E )和F( Imp-F );随后,根据 ICH M7 指南对杂质进行评估和分类。采用两个基于专家规则和统计的互补定量构效关系(QSAR)评价系统(Derek和Sarah)初步预测Imp-E和Imp-F的遗传毒性,E的预测结果疑似阳性。 Imp-E和Imp-F的Ames试验中,在每板62.5~1000μg的剂量范围内,有或没有代谢活化系统S9存在时,回复菌落数不超过菌落数的2倍溶剂对照组未表现出剂量反应关系,试验结果呈阴性。 Imp-E和Imp-F经测定遗传毒性呈阴性,可控制为ICH M7中的5类,即非致突变杂质。















































 京公网安备 11010802027423号
京公网安备 11010802027423号