当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic studies on Cu(NO3)2/TEMPO-catalyzed aerobic oxidation of alcohols to carboxylic acids
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-08 , DOI: 10.1039/d4qo00791c Yibo Yu 1 , Zhao Sun 2 , Yin-Long Guo 2 , Xue Zhang 2 , Hui Qian 1 , Shengming Ma 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-08 , DOI: 10.1039/d4qo00791c Yibo Yu 1 , Zhao Sun 2 , Yin-Long Guo 2 , Xue Zhang 2 , Hui Qian 1 , Shengming Ma 1, 2
Affiliation
|
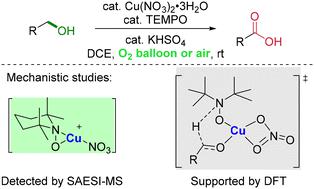
Oxidation of alcohols to carbonyl compounds is one of the most important and fundamental reactions in organic chemistry. Such aerobic reactions that provide aldehydes or ketones mediated by copper and nitroxyl have been well developed during the past several decades. Recently, we successfully realized the aerobic oxidation of primary alcohols to carboxylic acids catalyzed by Cu/TEMPO for the first time. Herein, a mechanism involving a Cu(NO3)2–TEMPO adduct as identified by SAESI-MS studies is proposed, which is supported by our experimental studies and density functional theory (DFT) calculations.
中文翻译:

Cu(NO3)2/TEMPO催化醇有氧氧化生成羧酸的机理研究
醇氧化成羰基化合物是有机化学中最重要和基本的反应之一。这种由铜和硝酰基介导的提供醛或酮的需氧反应在过去几十年中得到了很好的发展。最近,我们首次成功实现了Cu/TEMPO催化伯醇有氧氧化生成羧酸。在此,提出了通过 SAESI-MS 研究确定的涉及 Cu(NO 3 ) 2 –TEMPO 加合物的机制,该机制得到了我们的实验研究和密度泛函理论 (DFT) 的支持。 )计算。
更新日期:2024-07-08
中文翻译:

Cu(NO3)2/TEMPO催化醇有氧氧化生成羧酸的机理研究
醇氧化成羰基化合物是有机化学中最重要和基本的反应之一。这种由铜和硝酰基介导的提供醛或酮的需氧反应在过去几十年中得到了很好的发展。最近,我们首次成功实现了Cu/TEMPO催化伯醇有氧氧化生成羧酸。在此,提出了通过 SAESI-MS 研究确定的涉及 Cu(NO 3 ) 2 –TEMPO 加合物的机制,该机制得到了我们的实验研究和密度泛函理论 (DFT) 的支持。 )计算。












































 京公网安备 11010802027423号
京公网安备 11010802027423号