当前位置:
X-MOL 学术
›
Chem. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Weathering-induced Sb isotope fractionation during leaching of stibnite and formation of secondary Sb minerals
Chemical Geology ( IF 3.6 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.chemgeo.2024.122253 Andreas B. Kaufmann , Marina Lazarov , Ingo Horn , Martin Števko , Tamara Ðorđević , Stefan Kiefer , Stefan Weyer , Juraj Majzlan
Chemical Geology ( IF 3.6 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.chemgeo.2024.122253 Andreas B. Kaufmann , Marina Lazarov , Ingo Horn , Martin Števko , Tamara Ðorđević , Stefan Kiefer , Stefan Weyer , Juraj Majzlan
|
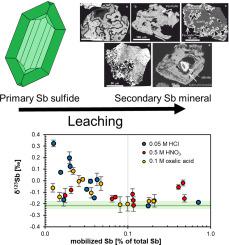
In this work, we investigated the extent of antimony (Sb) isotopic fractionation during weathering of stibnite at supergene conditions. Antimony isotope data have been obtained from secondary Sb minerals collected from Pezinok, Dobšiná (both Slovakia) and Allchar (North Macedonia) deposits and mine tailings. The Sb isotope compositions of sulfides and secondary Sb minerals formed on the primary stibnite [SbS] or in mine tailings grains were compared with each other. Furthermore, we experimentally investigated Sb isotope fractionation during stibnite leaching with different acids. Our study reveals a large isotopic range for δSb (from −0.50 to +0.69 ‰) for secondary Sb minerals. They are either isotopically indistinguishable or isotopically lighter than the primary stibnite. Isotopically indistinguishable weathering products likely formed by quantitative Sb transfer from stibnite to the secondary minerals, such as brandholzite [Mg(HO)[Sb(OH)]] from Pezinok. Isotopic fractionation towards lighter δSb was observed for adsorption of Sb onto iron oxides. Distinctly isotopically lighter δSb was observed in secondary Sb minerals tripuhyite [FeSbO], chapmanite [FeSb(SiO)O(OH)], hydroxyferroromeite [FeSbO(OH)], and stibiconite [SbOOH] that either replace stibnite or formed in mine tailings from the pore solutions. These secondary minerals were likely generated by partial precipitation of Sb from aqueous solutions produced by dissolution of stibnite. In the leaching experiments with HCl and oxalic acid, Sb was leached without significant isotope effects during the first 2–3 days, followed by a drop of the dissolved Sb concentration associated with Sb isotope fractionation towards high δSb in the leachate (by up to 0.5 ‰) after 4–7 days. We interpret these observations to be related to the precipitation of secondary Sb oxides with low δSb, resulting in an isotopically heavy dissolved Sb pool. These findings are in agreement with previous results of isotopically heavy groundwater and mine drainage water with δSb > +0.36 ‰ that may suggest that the ‘truly’ dissolved (operationally defined as <0.45 μm) Sb fraction in general may be isotopically heavy.
中文翻译:

辉锑矿浸出过程中风化引起的锑同位素分馏和次生锑矿物的形成
在这项工作中,我们研究了表生条件下辉锑矿风化过程中锑 (Sb) 同位素分馏的程度。锑同位素数据是从 Pezinok、Dobšiná(均为斯洛伐克)和 Allchar(北马其顿)矿床和尾矿收集的次生锑矿物中获得的。对原生辉锑矿 [SbS] 或尾矿颗粒中形成的硫化物和次生锑矿物的锑同位素组成进行了相互比较。此外,我们还通过实验研究了不同酸浸出辉锑矿过程中的锑同位素分馏。我们的研究揭示了次生锑矿物的 δSb 同位素范围较大(从 -0.50 到 +0.69 ‰)。它们要么在同位素上无法区分,要么在同位素上比原生辉锑矿轻。同位素无法区分的风化产物可能是由定量锑从辉锑矿转移到次生矿物而形成的,例如来自 Pezinok 的牌辉石 [Mg(H2O)[Sb(OH)]]。观察到 Sb 吸附到氧化铁上时,同位素分馏朝向较轻的 δSb。在次生锑矿物三辉石 [FeSbO]、菱铁矿 [FeSb(SiO)O(OH)]、羟基铁镁石 [FeSbO(OH)] 和辉锑矿 [SbOOH] 中观察到同位素明显较轻的 δSb,这些矿物要么取代辉锑矿,要么在尾矿中形成孔隙溶液。这些次生矿物可能是由辉锑矿溶解产生的水溶液中锑的部分沉淀产生的。在使用 HCl 和草酸的浸出实验中,在前 2-3 天内,锑的浸出没有明显的同位素效应,随后与锑同位素分馏相关的溶解锑浓度下降至浸出液中的高 δSb(最多 0.5 ‰) 4-7 天后。 我们将这些观察结果解释为与具有低 δSb 的次生锑氧化物的沉淀有关,从而导致同位素重的溶解锑池。这些发现与之前对重同位素地下水和矿井排水(δSb > +0.36 ‰)的结果一致,这可能表明“真正”溶解(操作上定义为 <0.45 μm)的锑部分通常可能是重同位素的。
更新日期:2024-06-25
中文翻译:

辉锑矿浸出过程中风化引起的锑同位素分馏和次生锑矿物的形成
在这项工作中,我们研究了表生条件下辉锑矿风化过程中锑 (Sb) 同位素分馏的程度。锑同位素数据是从 Pezinok、Dobšiná(均为斯洛伐克)和 Allchar(北马其顿)矿床和尾矿收集的次生锑矿物中获得的。对原生辉锑矿 [SbS] 或尾矿颗粒中形成的硫化物和次生锑矿物的锑同位素组成进行了相互比较。此外,我们还通过实验研究了不同酸浸出辉锑矿过程中的锑同位素分馏。我们的研究揭示了次生锑矿物的 δSb 同位素范围较大(从 -0.50 到 +0.69 ‰)。它们要么在同位素上无法区分,要么在同位素上比原生辉锑矿轻。同位素无法区分的风化产物可能是由定量锑从辉锑矿转移到次生矿物而形成的,例如来自 Pezinok 的牌辉石 [Mg(H2O)[Sb(OH)]]。观察到 Sb 吸附到氧化铁上时,同位素分馏朝向较轻的 δSb。在次生锑矿物三辉石 [FeSbO]、菱铁矿 [FeSb(SiO)O(OH)]、羟基铁镁石 [FeSbO(OH)] 和辉锑矿 [SbOOH] 中观察到同位素明显较轻的 δSb,这些矿物要么取代辉锑矿,要么在尾矿中形成孔隙溶液。这些次生矿物可能是由辉锑矿溶解产生的水溶液中锑的部分沉淀产生的。在使用 HCl 和草酸的浸出实验中,在前 2-3 天内,锑的浸出没有明显的同位素效应,随后与锑同位素分馏相关的溶解锑浓度下降至浸出液中的高 δSb(最多 0.5 ‰) 4-7 天后。 我们将这些观察结果解释为与具有低 δSb 的次生锑氧化物的沉淀有关,从而导致同位素重的溶解锑池。这些发现与之前对重同位素地下水和矿井排水(δSb > +0.36 ‰)的结果一致,这可能表明“真正”溶解(操作上定义为 <0.45 μm)的锑部分通常可能是重同位素的。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号