当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption and separation of lead ions in phosphoric acid by co-doped carbon nanotubes with sulfur, oxygen, and manganese
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2024-06-28 , DOI: 10.1016/j.jiec.2024.06.029 Gang Cheng , Xin Zhou , Chongyu Du , Guotao Hu , Qian Lin , Hongyan Pan
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2024-06-28 , DOI: 10.1016/j.jiec.2024.06.029 Gang Cheng , Xin Zhou , Chongyu Du , Guotao Hu , Qian Lin , Hongyan Pan
|
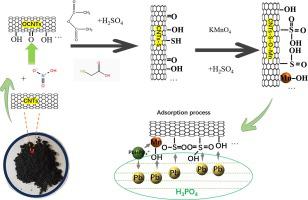
The purpose of this study was to prepare a novel and efficient adsorbent for the removal of trace Pb(II) from phosphoric acid. Manganese oxide/sulfur-oxygen doped carbon nanotube composites (CNTs-S-O-Mn) were prepared from multi-walled carbon nanotubes modified with nitric acid, mercaptoacetic acid, acetic anhydride, concentrated sulfuric acid and potassium permanganate. Characterisation revealed that CNTs-S-O-Mn contained hydroxyl, sulphonate groups, MnO and MnO. At 298 K, 15.362 mg·L initial concentration of lead ions, and 18.4 % phosphoric acid concentration, the adsorption capacity of the composites for lead ions was 38.65 mg·g, which was higher than that of the unmodified CNTs, 8.04 mg·g. The adsorption kinetic data at 298 K conformed to the quasi-second-order kinetic equation, R = 0.997; 298, adsorption isotherms at 308 and 318 K conformed to the Langmuir equation, R = 0.990–––0.991. The adsorption capacity increased with decreasing phosphoric acid concentration. In this system, the adsorption is affected by a certain concentration of lead ions. Adsorption occurs via chemisorption and is exothermic. Sulfonic acid groups and hydroxyl groups on manganese oxides play an important role in the adsorption of Pb (II) through surface complexation with Pb (II). These results indicate that the removal of trace Pb (II) from phosphoric acid by modified carbon nanotubes is feasible, revealing a new use of carbon nanotubes for phosphoric acid purification.
中文翻译:

硫、氧、锰共掺杂碳纳米管对磷酸中铅离子的吸附分离
本研究的目的是制备一种新型高效吸附剂,用于去除磷酸中的痕量 Pb(II)。以硝酸、巯基乙酸、醋酐、浓硫酸和高锰酸钾改性的多壁碳纳米管为原料,制备了氧化锰/硫氧掺杂碳纳米管复合材料(CNTs-S-O-Mn)。表征表明CNTs-S-O-Mn含有羟基、磺酸盐基团、MnO和MnO。在298 K、铅离子初始浓度15.362 mg·L、磷酸浓度18.4%时,复合材料对铅离子的吸附容量为38.65 mg·g,高于未改性CNT的8.04 mg·g。 。 298 K下的吸附动力学数据符合准二级动力学方程,R = 0.997; 298,308和318 K的吸附等温线符合Langmuir方程,R = 0.990–––0.991。吸附容量随着磷酸浓度的降低而增加。在该系统中,吸附受到一定浓度的铅离子的影响。吸附通过化学吸附发生并且是放热的。锰氧化物上的磺酸基和羟基通过与 Pb(II) 的表面络合,在吸附 Pb(II) 方面发挥着重要作用。这些结果表明,改性碳纳米管去除磷酸中痕量Pb(II)是可行的,揭示了碳纳米管用于磷酸纯化的新用途。
更新日期:2024-06-28
中文翻译:

硫、氧、锰共掺杂碳纳米管对磷酸中铅离子的吸附分离
本研究的目的是制备一种新型高效吸附剂,用于去除磷酸中的痕量 Pb(II)。以硝酸、巯基乙酸、醋酐、浓硫酸和高锰酸钾改性的多壁碳纳米管为原料,制备了氧化锰/硫氧掺杂碳纳米管复合材料(CNTs-S-O-Mn)。表征表明CNTs-S-O-Mn含有羟基、磺酸盐基团、MnO和MnO。在298 K、铅离子初始浓度15.362 mg·L、磷酸浓度18.4%时,复合材料对铅离子的吸附容量为38.65 mg·g,高于未改性CNT的8.04 mg·g。 。 298 K下的吸附动力学数据符合准二级动力学方程,R = 0.997; 298,308和318 K的吸附等温线符合Langmuir方程,R = 0.990–––0.991。吸附容量随着磷酸浓度的降低而增加。在该系统中,吸附受到一定浓度的铅离子的影响。吸附通过化学吸附发生并且是放热的。锰氧化物上的磺酸基和羟基通过与 Pb(II) 的表面络合,在吸附 Pb(II) 方面发挥着重要作用。这些结果表明,改性碳纳米管去除磷酸中痕量Pb(II)是可行的,揭示了碳纳米管用于磷酸纯化的新用途。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号