当前位置:
X-MOL 学术
›
J. Environ. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Removal of cyanide by peroxydisulfate activated by copper ions inherently present in the electroplating wastewater
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2024-07-06 , DOI: 10.1016/j.jece.2024.113517
Zih-Syuan Wang , Yi-Chin Cho , Yi-Pin Lin
Journal of Environmental Chemical Engineering ( IF 7.4 ) Pub Date : 2024-07-06 , DOI: 10.1016/j.jece.2024.113517
Zih-Syuan Wang , Yi-Chin Cho , Yi-Pin Lin
|
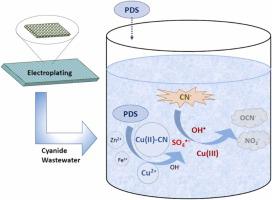
Cyanide is widely used in electroplating processes to ensure the quality of metal plating. Alkaline chlorination process is commonly employed to remove cyanide from electroplating wastewater. However, several drawbacks exist, including the formation of toxic intermediates, high consumption of chemical reagents, and low removal efficiency of metal-cyanide complexes. In this study, persulfate advanced oxidation process was explored for the removal of cyanide from electroplating wastewater. The metal ions typically present in the electroplating wastewater, including Cu2+ , Zn2+ , Fe2+ , Ag+ and Ni2+ , may activate peroxydisulfate (PDS) to oxidize cyanide. Among these metal ions, cyanide can be most effectively removed in the presence of Cu2+ and PDS, likely due to the activation of PDS by Cu2+ and Cu(II)-cyanide complex. The results of lab studies indicated that 99 % removal of cyanide (4 mM) could be achieved in the presence of 10 mM PDS and 1 mM Cu2+ within 20 min. The results of radical scavenging experiments, radical probe tests and electron paramagnetic resonance (EPR) studies showed that sulfate radical (SO4 •− ) and hydroxyl radical (OH• ) were primarily responsible for cyanide removal and the complexation of cyanide and Cu2+ significantly enhanced the formation of SO4 •− . Cyanate and nitrite were the main by-products detected. For real electroplating wastewater, the addition of 40 mM PDS could almost completely remove cyanide (19.5 mM) in 20 min.
中文翻译:

通过电镀废水中固有的铜离子活化的过氧二硫酸盐去除氰化物
氰化物广泛用于电镀工艺,以保证金属镀层的质量。碱性氯化工艺通常用于去除电镀废水中的氰化物。然而,也存在一些缺点,包括形成有毒中间体、化学试剂消耗量大、金属氰化物络合物去除效率低。本研究探讨了过硫酸盐高级氧化工艺去除电镀废水中的氰化物。电镀废水中通常存在的金属离子,包括 Cu2+、Zn2+、Fe2+、Ag+ 和 Ni2+,可能会激活过氧化物二硫酸盐 (PDS) 以氧化氰化物。在这些金属离子中,在 Cu2+ 和 PDS 存在下可以最有效地去除氰化物,这可能是由于 Cu2+ 和 Cu(II)-氰化物络合物激活了 PDS。实验室研究结果表明,在 10 mM PDS 和 1 mM Cu2+ 存在下,可在 20 分钟内实现 99% 的氰化物去除 (4 mM)。自由基清除实验、自由基探针测试和电子顺磁共振 (EPR) 研究结果表明,硫酸根自由基 (SO4•−) 和羟基自由基 (OH•) 是去除氰化物的主要原因,氰化物和 Cu2+ 的络合显著促进了 SO4•− 的形成。氰酸盐和亚硝酸盐是检测到的主要副产物。对于真正的电镀废水,添加 40 mM PDS 几乎可以在 20 分钟内完全去除氰化物 (19.5 mM)。
更新日期:2024-07-06
中文翻译:

通过电镀废水中固有的铜离子活化的过氧二硫酸盐去除氰化物
氰化物广泛用于电镀工艺,以保证金属镀层的质量。碱性氯化工艺通常用于去除电镀废水中的氰化物。然而,也存在一些缺点,包括形成有毒中间体、化学试剂消耗量大、金属氰化物络合物去除效率低。本研究探讨了过硫酸盐高级氧化工艺去除电镀废水中的氰化物。电镀废水中通常存在的金属离子,包括 Cu2+、Zn2+、Fe2+、Ag+ 和 Ni2+,可能会激活过氧化物二硫酸盐 (PDS) 以氧化氰化物。在这些金属离子中,在 Cu2+ 和 PDS 存在下可以最有效地去除氰化物,这可能是由于 Cu2+ 和 Cu(II)-氰化物络合物激活了 PDS。实验室研究结果表明,在 10 mM PDS 和 1 mM Cu2+ 存在下,可在 20 分钟内实现 99% 的氰化物去除 (4 mM)。自由基清除实验、自由基探针测试和电子顺磁共振 (EPR) 研究结果表明,硫酸根自由基 (SO4•−) 和羟基自由基 (OH•) 是去除氰化物的主要原因,氰化物和 Cu2+ 的络合显著促进了 SO4•− 的形成。氰酸盐和亚硝酸盐是检测到的主要副产物。对于真正的电镀废水,添加 40 mM PDS 几乎可以在 20 分钟内完全去除氰化物 (19.5 mM)。

































 京公网安备 11010802027423号
京公网安备 11010802027423号