Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic consequences of the identity and coverages of reactive intermediates during benzene hydrogenation on Pt, Pd, and Pt-Re catalysts
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-06-03 , DOI: 10.1016/j.jcat.2024.115582 Haiting Cai , Haoyu Nie , Zhuole Lu , Chandra Veer Singh , Ya-Huei (Cathy) Chin
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-06-03 , DOI: 10.1016/j.jcat.2024.115582 Haiting Cai , Haoyu Nie , Zhuole Lu , Chandra Veer Singh , Ya-Huei (Cathy) Chin
|
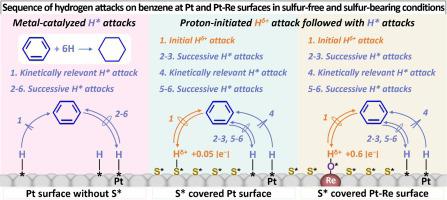
Mechanistic differences in benzene and cyclohexene hydrogenation on Pt, Pd, and Pt-Re catalysts are interrogated with kinetic assessments, temperature programmed surface reactions, infrared absorption spectroscopic study, and DFT calculations. Their effective rate constants are larger on Pt than Pd surfaces either uncovered or covered with sulfur species, due to the weaker H* adsorption and higher SH* acidity on Pt than Pd. Without sulfur species, H* is the only reactive hydrogen species—on Pt, its first insertion on the adsorbed benzene (CH*) and its second insertion onto the cyclohexene intermediate (CH*) restrict turnovers in benzene and cyclohexane hydrogenation, respectively. HS treatments lead to SH* species bound irreversibly that not only decreases turnovers but also alters the elementary steps and their kinetic relevance within the catalytic cycle—SH* initially attacks CH*, before five successive H* additions, where the third addition limits turnovers. Incorporating isolated Re atoms onto sulfur-covered Pt surfaces increases benzene hydrogenation turnovers, because OH* associated with Re sites are more acidic and effective in initiating the reaction.
中文翻译:

Pt、Pd 和 Pt-Re 催化剂上苯加氢过程中反应中间体的特性和覆盖率的催化结果
通过动力学评估、程序升温表面反应、红外吸收光谱研究和 DFT 计算,探讨了 Pt、Pd 和 Pt-Re 催化剂上苯和环己烯加氢的机理差异。无论是未覆盖的还是被硫物质覆盖的表面,它们在 Pt 上的有效速率常数都大于 Pd 表面,因为 Pt 上的 H* 吸附较弱,SH* 酸性较高。在没有硫物质的情况下,H* 是 Pt 上唯一的活性氢物质,它在吸附的苯 (CH*) 上的第一次插入和在环己烯中间体 (CH*) 上的第二次插入分别限制了苯和环己烷氢化的转换。 HS处理导致SH*物质不可逆地结合,这不仅降低了周转率,而且改变了催化循环中的基本步骤及其动力学相关性——SH*最初攻击CH*,然后连续五次添加H*,其中第三次添加限制了周转率。将孤立的 Re 原子结合到硫覆盖的 Pt 表面上可以增加苯氢化的转化率,因为与 Re 位点相关的 OH* 酸性更强,可以更有效地引发反应。
更新日期:2024-06-03
中文翻译:

Pt、Pd 和 Pt-Re 催化剂上苯加氢过程中反应中间体的特性和覆盖率的催化结果
通过动力学评估、程序升温表面反应、红外吸收光谱研究和 DFT 计算,探讨了 Pt、Pd 和 Pt-Re 催化剂上苯和环己烯加氢的机理差异。无论是未覆盖的还是被硫物质覆盖的表面,它们在 Pt 上的有效速率常数都大于 Pd 表面,因为 Pt 上的 H* 吸附较弱,SH* 酸性较高。在没有硫物质的情况下,H* 是 Pt 上唯一的活性氢物质,它在吸附的苯 (CH*) 上的第一次插入和在环己烯中间体 (CH*) 上的第二次插入分别限制了苯和环己烷氢化的转换。 HS处理导致SH*物质不可逆地结合,这不仅降低了周转率,而且改变了催化循环中的基本步骤及其动力学相关性——SH*最初攻击CH*,然后连续五次添加H*,其中第三次添加限制了周转率。将孤立的 Re 原子结合到硫覆盖的 Pt 表面上可以增加苯氢化的转化率,因为与 Re 位点相关的 OH* 酸性更强,可以更有效地引发反应。












































 京公网安备 11010802027423号
京公网安备 11010802027423号