当前位置:
X-MOL 学术
›
Adv. Drug Deliver. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Model-informed drug development in pediatric, pregnancy and geriatric drug development: States of the art and future
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.addr.2024.115364 Yue-E Wu 1 , Yuan-Yuan Zheng 1 , Qiu-Yue Li 1 , Bu-Fan Yao 1 , Jing Cao 1 , Hui-Xin Liu 1 , Guo-Xiang Hao 1 , John van den Anker 2 , Yi Zheng 1 , Wei Zhao 1
Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2024-06-25 , DOI: 10.1016/j.addr.2024.115364 Yue-E Wu 1 , Yuan-Yuan Zheng 1 , Qiu-Yue Li 1 , Bu-Fan Yao 1 , Jing Cao 1 , Hui-Xin Liu 1 , Guo-Xiang Hao 1 , John van den Anker 2 , Yi Zheng 1 , Wei Zhao 1
Affiliation
|
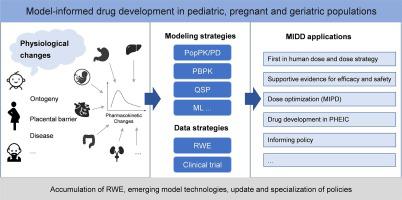
The challenges of drug development in pediatric, pregnant and geriatric populations are a worldwide concern shared by regulatory authorities, pharmaceutical companies, and healthcare professionals. Model-informed drug development (MIDD) can integrate and quantify real-world data of physiology, pharmacology, and disease processes by using modeling and simulation techniques to facilitate decision-making in drug development. In this article, we reviewed current MIDD policy updates, reflected on the integrity of physiological data used for MIDD and the effects of physiological changes on the drug PK, as well as summarized current MIDD strategies and applications, so as to present the state of the art of MIDD in pediatric, pregnant and geriatric populations. Some considerations are put forth for the future improvements of MIDD including refining regulatory considerations, improving the integrity of physiological data, applying the emerging technologies, and exploring the application of MIDD in new therapies like gene therapies for special populations.
中文翻译:

儿科、妊娠和老年药物开发中基于模型的药物开发:最新技术和未来
儿科、孕妇和老年人群药物开发的挑战是全球监管机构、制药公司和医疗保健专业人员共同关注的问题。模型知情药物开发(MIDD)可以通过使用建模和模拟技术来整合和量化生理学、药理学和疾病过程的真实世界数据,以促进药物开发决策。在本文中,我们回顾了当前 MIDD 政策的更新,反思了用于 MIDD 的生理数据的完整性以及生理变化对药物 PK 的影响,并总结了当前 MIDD 策略和应用,以呈现 MIDD 的现状MIDD 在儿科、孕妇和老年人群中的艺术。对MIDD的未来改进提出了一些考虑,包括细化监管考虑、提高生理数据的完整性、应用新兴技术以及探索MIDD在特殊人群基因治疗等新疗法中的应用。
更新日期:2024-06-25
中文翻译:

儿科、妊娠和老年药物开发中基于模型的药物开发:最新技术和未来
儿科、孕妇和老年人群药物开发的挑战是全球监管机构、制药公司和医疗保健专业人员共同关注的问题。模型知情药物开发(MIDD)可以通过使用建模和模拟技术来整合和量化生理学、药理学和疾病过程的真实世界数据,以促进药物开发决策。在本文中,我们回顾了当前 MIDD 政策的更新,反思了用于 MIDD 的生理数据的完整性以及生理变化对药物 PK 的影响,并总结了当前 MIDD 策略和应用,以呈现 MIDD 的现状MIDD 在儿科、孕妇和老年人群中的艺术。对MIDD的未来改进提出了一些考虑,包括细化监管考虑、提高生理数据的完整性、应用新兴技术以及探索MIDD在特殊人群基因治疗等新疗法中的应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号