Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-07-04 , DOI: 10.1002/adsc.202400538 Neng-Quan Jiang 1 , Ming-Ming Li 2 , Li-Jun Xiao 2 , Qi-Lin Zhou 2
|
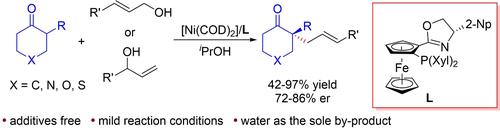
Transition-metal-catalyzed asymmetric α-allylation of ketones is a particularly useful tool for constructing stereocenters via C−C bond formation. However, this type of reaction often necessitated the use of activated ketones or pre-prepared enol intermediates as substrates. In this study, we developed a nickel-catalyzed α-allylation of unactivated cycloketones with allylic alcohols to synthesize chiral cycloketones with an α-quaternary carbon center. The reaction affords the products with 42–97% yields, 72–86% ee, and no additive is needed, and the only by-product is water.
中文翻译:

镍催化环酮与烯丙醇的对映选择性 α-烯丙基化
过渡金属催化的酮的不对称 α-烯丙基化是通过 C−C 键形成构建立体中心的特别有用的工具。然而,此类反应通常需要使用活化的酮或预先制备的烯醇中间体作为底物。在这项研究中,我们开发了未活化环酮与烯丙醇的镍催化α-烯丙基化反应,以合成具有α-季碳中心的手性环酮。该反应产物收率42-97%,ee 72-86%,不需要任何添加剂,唯一的副产物是水。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号