当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
More than an Amide Bioisostere: Discovery of 1,2,4-Triazole-containing Pyrazolo[1,5-a]pyrimidine Host CSNK2 Inhibitors for Combatting β-Coronavirus Replication
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-07-03 , DOI: 10.1021/acs.jmedchem.4c00962 Han Wee Ong 1, 2 , Xuan Yang 1, 2 , Jeffery L Smith 2 , Rebekah J Dickmander 1, 3, 4, 5 , Jason W Brown 6 , Tammy M Havener 2 , Sharon Taft-Benz 1, 7 , Stefanie Howell 2 , Marcia K Sanders 1, 7 , Jacob L Capener 2 , Rafael M Couñago 2, 8 , Edcon Chang 6 , Andreas Krämer 9 , Nathaniel J Moorman 1, 3, 4 , Mark Heise 1, 7 , Alison D Axtman 1, 2 , David H Drewry 1, 2, 4 , Timothy M Willson 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-07-03 , DOI: 10.1021/acs.jmedchem.4c00962 Han Wee Ong 1, 2 , Xuan Yang 1, 2 , Jeffery L Smith 2 , Rebekah J Dickmander 1, 3, 4, 5 , Jason W Brown 6 , Tammy M Havener 2 , Sharon Taft-Benz 1, 7 , Stefanie Howell 2 , Marcia K Sanders 1, 7 , Jacob L Capener 2 , Rafael M Couñago 2, 8 , Edcon Chang 6 , Andreas Krämer 9 , Nathaniel J Moorman 1, 3, 4 , Mark Heise 1, 7 , Alison D Axtman 1, 2 , David H Drewry 1, 2, 4 , Timothy M Willson 1, 2
Affiliation
|
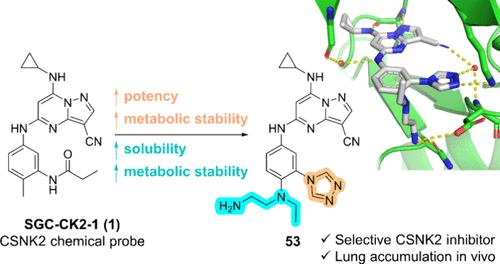
The pyrazolo[1,5-a]pyrimidine scaffold is a promising scaffold to develop potent and selective CSNK2 inhibitors with antiviral activity against β-coronaviruses. Herein, we describe the discovery of a 1,2,4-triazole group to substitute a key amide group for CSNK2 binding present in many potent pyrazolo[1,5-a]pyrimidine inhibitors. Crystallographic evidence demonstrates that the 1,2,4-triazole replaces the amide in forming key hydrogen bonds with Lys68 and a water molecule buried in the ATP-binding pocket. This isosteric replacement improves potency and metabolic stability at a cost of solubility. Optimization for potency, solubility, and metabolic stability led to the discovery of the potent and selective CSNK2 inhibitor 53. Despite excellent in vitro metabolic stability, rapid decline in plasma concentration of 53 in vivo was observed and may be attributed to lung accumulation, although in vivo pharmacological effect was not observed. Further optimization of this novel chemotype may validate CSNK2 as an antiviral target in vivo.
中文翻译:

不仅仅是酰胺生物等排体:发现含有 1,2,4-三唑的吡唑并[1,5-a]嘧啶宿主 CSNK2 抑制剂,用于对抗 β-冠状病毒复制
吡唑并[1,5- a ]嘧啶支架是一种很有前途的支架,可用于开发具有抗β-冠状病毒活性的有效、选择性CSNK2抑制剂。在此,我们描述了 1,2,4-三唑基团的发现,该基团取代了许多有效的吡唑并[1,5- a ]嘧啶抑制剂中存在的 CSNK2 结合的关键酰胺基团。晶体学证据表明,1,2,4-三唑取代了酰胺,与 Lys68 和埋在 ATP 结合袋中的水分子形成关键氢键。这种等排替代以溶解度为代价提高了效力和代谢稳定性。效力、溶解度和代谢稳定性的优化导致了有效且选择性 CSNK2 抑制剂的发现53 。尽管具有优异的体外代谢稳定性,但在体内观察到53的血浆浓度快速下降,并且可能归因于肺蓄积,尽管没有观察到体内药理作用。这种新型化学型的进一步优化可能会验证 CSNK2 作为体内抗病毒靶点。
更新日期:2024-07-03
中文翻译:

不仅仅是酰胺生物等排体:发现含有 1,2,4-三唑的吡唑并[1,5-a]嘧啶宿主 CSNK2 抑制剂,用于对抗 β-冠状病毒复制
吡唑并[1,5- a ]嘧啶支架是一种很有前途的支架,可用于开发具有抗β-冠状病毒活性的有效、选择性CSNK2抑制剂。在此,我们描述了 1,2,4-三唑基团的发现,该基团取代了许多有效的吡唑并[1,5- a ]嘧啶抑制剂中存在的 CSNK2 结合的关键酰胺基团。晶体学证据表明,1,2,4-三唑取代了酰胺,与 Lys68 和埋在 ATP 结合袋中的水分子形成关键氢键。这种等排替代以溶解度为代价提高了效力和代谢稳定性。效力、溶解度和代谢稳定性的优化导致了有效且选择性 CSNK2 抑制剂的发现53 。尽管具有优异的体外代谢稳定性,但在体内观察到53的血浆浓度快速下降,并且可能归因于肺蓄积,尽管没有观察到体内药理作用。这种新型化学型的进一步优化可能会验证 CSNK2 作为体内抗病毒靶点。































 京公网安备 11010802027423号
京公网安备 11010802027423号