当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Methanolysis of PET Catalyzed by Nonmetallic Deep Eutectic Solvents
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-07-02 , DOI: 10.1021/acs.iecr.4c00720 Jiabao Li 1, 2 , Dongxia Yan 2, 3 , Xiujie Cheng 2, 3 , Chunrui Rong 2, 4 , Jian Feng 2, 5 , Xin Feng 1 , Jiayu Xin 2, 3 , Qing Zhou 2, 3 , Yi Li 2, 3 , Junli Xu 2, 3 , Xingmei Lu 2, 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-07-02 , DOI: 10.1021/acs.iecr.4c00720 Jiabao Li 1, 2 , Dongxia Yan 2, 3 , Xiujie Cheng 2, 3 , Chunrui Rong 2, 4 , Jian Feng 2, 5 , Xin Feng 1 , Jiayu Xin 2, 3 , Qing Zhou 2, 3 , Yi Li 2, 3 , Junli Xu 2, 3 , Xingmei Lu 2, 3
Affiliation
|
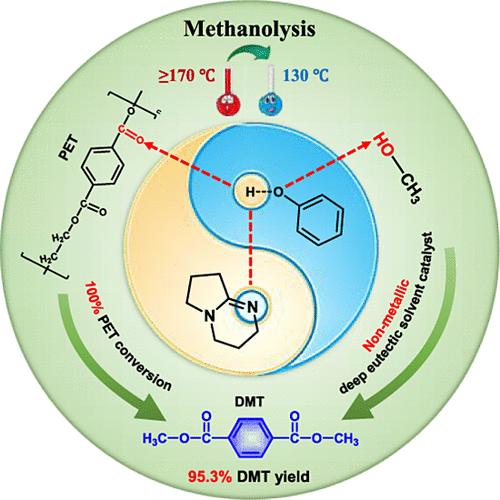
The methanolysis of polyethylene terephthalate (PET) can address the environmental pollution and resource waste caused by waste PET, and the obtained monomer dimethyl terephthalate (DMT) is easy to separate and purify. However, conventional methanolysis of PET usually uses metal-containing catalysts, resulting in metal residues in the degradation products, which affects subsequent high-value utilization. Meantime, the reaction conditions of high temperature and high pressure promotes the occurrence of side reactions and reduces the yield of the main products. Herein, the nonmetallic deep eutectic solvent (DES) 1,5-diazabicyclo [4.3.0]-5-nonene (DBN)/Phenol was employed as a catalyst for PET methanolysis. 100% PET conversion and 95.3% DMT yield were obtained at 130 °C for 1 h. The possible reaction mechanism was investigated by combining NMR, FT-IR, GPC analysis, and DFT calculations. DBN and phenol were connected through N–H–O hydrogen bonding, where the hydrogen atom interacted with the carbonyl oxygen of PET and the oxygen atom activated the hydroxyl group of methanol. Additionally, GPC analysis showed that the dissolution and degradation of PET were simultaneous, which enhanced mass transfer and facilitated the reaction. The kinetic studies showed that PET methanolysis followed the first-order kinetic model with an activation energy of 103.3 kJ/mol, which was lower than the literature-reported value. This research provides an efficient nonmetallic deep eutectic solvent catalyst for PET methanolysis, which solves the problem of metal residues in the degradation products and reduces the reaction temperature. It is of great significance to simplify the purification steps and improve the economy of regeneration.
中文翻译:

非金属低共熔溶剂催化 PET 高效甲醇分解
聚对苯二甲酸乙二醇酯(PET)的甲醇解可解决废旧PET造成的环境污染和资源浪费,且所得单体对苯二甲酸二甲酯(DMT)易于分离提纯。然而,传统的PET甲醇解通常使用含金属的催化剂,导致降解产物中存在金属残留,影响后续的高值利用。同时,高温高压的反应条件促进了副反应的发生,降低了主要产物的收率。在此,采用非金属低共熔溶剂(DES)1,5-二氮杂双环[4.3.0]-5-壬烯(DBN)/苯酚作为PET甲醇解的催化剂。在 130 °C 1 小时内获得 100% PET 转化率和 95.3% DMT 产率。通过结合NMR、FT-IR、GPC分析和DFT计算研究了可能的反应机理。 DBN和苯酚通过N-H-O氢键连接,其中氢原子与PET的羰基氧相互作用,氧原子激活甲醇的羟基。此外,GPC分析表明PET的溶解和降解是同时进行的,这增强了传质并促进了反应。动力学研究表明,PET甲醇解遵循一级动力学模型,活化能为103.3 kJ/mol,低于文献报道值。该研究为PET甲醇解提供了一种高效的非金属低共熔溶剂催化剂,解决了降解产物中金属残留的问题,并降低了反应温度。对简化纯化步骤、提高再生经济性具有重要意义。
更新日期:2024-07-02
中文翻译:

非金属低共熔溶剂催化 PET 高效甲醇分解
聚对苯二甲酸乙二醇酯(PET)的甲醇解可解决废旧PET造成的环境污染和资源浪费,且所得单体对苯二甲酸二甲酯(DMT)易于分离提纯。然而,传统的PET甲醇解通常使用含金属的催化剂,导致降解产物中存在金属残留,影响后续的高值利用。同时,高温高压的反应条件促进了副反应的发生,降低了主要产物的收率。在此,采用非金属低共熔溶剂(DES)1,5-二氮杂双环[4.3.0]-5-壬烯(DBN)/苯酚作为PET甲醇解的催化剂。在 130 °C 1 小时内获得 100% PET 转化率和 95.3% DMT 产率。通过结合NMR、FT-IR、GPC分析和DFT计算研究了可能的反应机理。 DBN和苯酚通过N-H-O氢键连接,其中氢原子与PET的羰基氧相互作用,氧原子激活甲醇的羟基。此外,GPC分析表明PET的溶解和降解是同时进行的,这增强了传质并促进了反应。动力学研究表明,PET甲醇解遵循一级动力学模型,活化能为103.3 kJ/mol,低于文献报道值。该研究为PET甲醇解提供了一种高效的非金属低共熔溶剂催化剂,解决了降解产物中金属残留的问题,并降低了反应温度。对简化纯化步骤、提高再生经济性具有重要意义。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号