当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Annulative Coupling of β-Ketosulfoxonium Ylides and β-Ketothioamides: Access to Thiazolines and Thiazolidin-4-Ones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-07-03 , DOI: 10.1002/adsc.202400581 Rahul Kumar Saini 1 , Satyendra Pandey 1
中文翻译:

β-酮磺铵酰化物和 β-酮硫代酰胺的环环偶联:噻唑啉和噻唑烷-4-酮的可及性
更新日期:2024-07-03
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-07-03 , DOI: 10.1002/adsc.202400581 Rahul Kumar Saini 1 , Satyendra Pandey 1
Affiliation
|
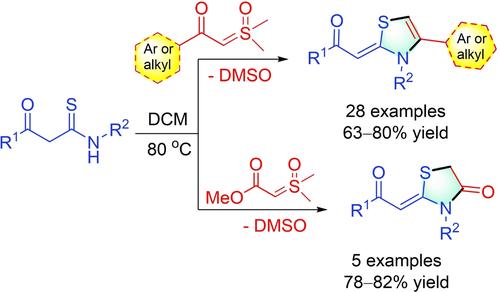
A catalyst- and additive-free one-step protocol for synthesizing thiazolines and thiazolidin-4-ones, utilizing the annulative coupling between β-ketosulfoxonium ylides and β-ketothioamides has been described. The dual functionalization of β-ketosulfoxonium ylides through S-alkylation and intramolecular N-cyclization leads to the generation of both C−S and C−N bonds. The developed synthetic methods facilitate the synthesis of diverse functionalized thiazolines and thiazolidin-4-one derivatives, validated through large-scale reactions.
中文翻译:

β-酮磺铵酰化物和 β-酮硫代酰胺的环环偶联:噻唑啉和噻唑烷-4-酮的可及性
已经描述了一种用于合成噻唑啉和噻唑烷-4-酮的一步法方案,利用β-酮磺酰鎓烯基化物和 β-酮硫代酰胺之间的年环偶联。β-酮磺铵酰化物通过 S-烷基化和分子内 N-环化作用的双重功能化导致 C-S 和 C-N 键的产生。开发的合成方法有助于合成多种功能化噻唑啉和噻唑烷-4-酮衍生物,并通过大规模反应验证。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号