当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalyzed dehalogenative deuteration with silacarboxylic acids as halogen-atom transfer agents
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-04 , DOI: 10.1039/d4qo01030b Jia-Wei Hu 1 , Jian Cao 1 , Li-Wen Xu 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-04 , DOI: 10.1039/d4qo01030b Jia-Wei Hu 1 , Jian Cao 1 , Li-Wen Xu 1, 2
Affiliation
|
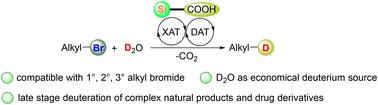
Deuterated chemicals are widely utilized in a broad range of disciplines and dehalogenative deuteration of organohalides represents a promising approach to obtaining deuterated chemicals. Herein, we disclose a visible light-induced organophotocatalytic strategy for dehalogenative deuteration of a wide variety of primary, secondary, and tertiary alkyl bromides. Key to this protocol is silyl radical-promoted halogen-atom transfer and thiol-mediated deuterium-atom transfer. Readily available silacarboxylic acids, which generate silyl radicals through efficient visible-light-induced decarboxylation, are applied as halogen-atom transfer agents. The protocol employs economical deuterium oxide as the source of deuterium. Late stage deuteration of complex natural products and drug derivatives demonstrated the potential of this environmentally benign method for practical applications.
中文翻译:

以硅羧酸作为卤素原子转移剂的光催化脱卤氘化
氘化化学品广泛应用于各个学科,有机卤化物的脱卤氘化是获得氘化化学品的一种有前景的方法。在此,我们公开了一种可见光诱导的有机光催化策略,用于多种伯、仲和叔烷基溴的脱卤氘化。该协议的关键是甲硅烷基促进的卤素原子转移和硫醇介导的氘原子转移。容易获得的硅羧酸可通过有效的可见光诱导脱羧作用产生甲硅烷基自由基,可用作卤素原子转移剂。该方案采用经济的氧化氘作为氘源。复杂天然产物和药物衍生物的后期氘化证明了这种环境友好方法在实际应用中的潜力。
更新日期:2024-07-04
中文翻译:

以硅羧酸作为卤素原子转移剂的光催化脱卤氘化
氘化化学品广泛应用于各个学科,有机卤化物的脱卤氘化是获得氘化化学品的一种有前景的方法。在此,我们公开了一种可见光诱导的有机光催化策略,用于多种伯、仲和叔烷基溴的脱卤氘化。该协议的关键是甲硅烷基促进的卤素原子转移和硫醇介导的氘原子转移。容易获得的硅羧酸可通过有效的可见光诱导脱羧作用产生甲硅烷基自由基,可用作卤素原子转移剂。该方案采用经济的氧化氘作为氘源。复杂天然产物和药物衍生物的后期氘化证明了这种环境友好方法在实际应用中的潜力。












































 京公网安备 11010802027423号
京公网安备 11010802027423号