Chem Catalysis ( IF 11.5 ) Pub Date : 2024-07-04 , DOI: 10.1016/j.checat.2024.101043 Shuang Yang , Qi Zhang , Hui Chen , Da Zhao , Yang Wang
|
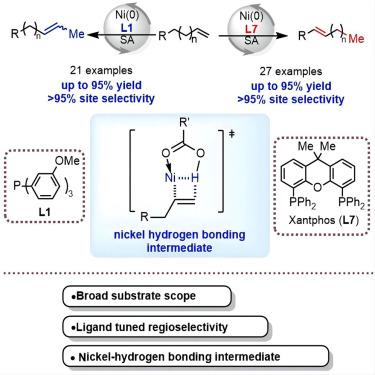
Olefin isomerization has an important impact on organic synthesis, fine chemicals, and polymer chemistry. However, due to the small energy differences between various potential isomers, achieving regioselective isomerization remains a challenge. We report on the nickel-catalyzed divergent isomerization of olefins, which leads to the selective formation of either a kinetic or thermodynamic isomer, with the specific product determined by the choice of phosphine ligand. This double-bond chain-walking reaction is characterized by its mild conditions, broad substrate scope, good regioselectivity, high yields, gram-scale synthesis, and the availability of materials from commercial sources. The pairing of this isomerization with ethenolysis led to the formation of formal one-carbon-deletion products. Mechanistic studies emphasize the critical role of the phosphine ligand in governing the regioselectivity of the double-bond chain-walking reaction, and a distinctive nickel-hydrogen-bonding intermediate was pointed out to be key to the success of this transformation.
中文翻译:

镍氢键作用、膦配体调节的烯烃区域发散异构化
烯烃异构化对有机合成、精细化工和高分子化学具有重要影响。然而,由于各种潜在异构体之间的能量差异较小,实现区域选择性异构化仍然是一个挑战。我们报道了镍催化的烯烃发散异构化,这导致选择性形成动力学或热力学异构体,具体产物由膦配体的选择决定。这种双键链行走反应具有条件温和、底物范围广、区域选择性好、收率高、克级规模合成以及商业来源的材料可得性等特点。这种异构化与乙烯醇分解的配对导致形成正式的一碳缺失产物。机理研究强调了膦配体在控制双键链行走反应的区域选择性中的关键作用,并且指出独特的镍氢键中间体是这种转化成功的关键。































 京公网安备 11010802027423号
京公网安备 11010802027423号