Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Progestogen-driven B7-H4 contributes to onco-fetal immune tolerance
Cell ( IF 45.5 ) Pub Date : 2024-07-04 , DOI: 10.1016/j.cell.2024.06.012
Jiali Yu 1 , Yijian Yan 1 , Shasha Li 1 , Ying Xu 1 , Abhijit Parolia 2 , Syed Rizvi 3 , Weichao Wang 1 , Yiwen Zhai 4 , Rongxin Xiao 1 , Xiong Li 1 , Peng Liao 1 , Jiajia Zhou 1 , Karolina Okla 5 , Heng Lin 1 , Xun Lin 1 , Sara Grove 1 , Shuang Wei 1 , Linda Vatan 1 , Jiantao Hu 6 , Justyna Szumilo 7 , Jan Kotarski 8 , Zachary T Freeman 9 , Stephanie Skala 2 , Max Wicha 6 , Kathleen R Cho 2 , Arul M Chinnaiyan 10 , Samantha Schon 11 , Fei Wen 3 , Ilona Kryczek 1 , Shaomeng Wang 12 , Lieping Chen 13 , Weiping Zou 14
Cell ( IF 45.5 ) Pub Date : 2024-07-04 , DOI: 10.1016/j.cell.2024.06.012
Jiali Yu 1 , Yijian Yan 1 , Shasha Li 1 , Ying Xu 1 , Abhijit Parolia 2 , Syed Rizvi 3 , Weichao Wang 1 , Yiwen Zhai 4 , Rongxin Xiao 1 , Xiong Li 1 , Peng Liao 1 , Jiajia Zhou 1 , Karolina Okla 5 , Heng Lin 1 , Xun Lin 1 , Sara Grove 1 , Shuang Wei 1 , Linda Vatan 1 , Jiantao Hu 6 , Justyna Szumilo 7 , Jan Kotarski 8 , Zachary T Freeman 9 , Stephanie Skala 2 , Max Wicha 6 , Kathleen R Cho 2 , Arul M Chinnaiyan 10 , Samantha Schon 11 , Fei Wen 3 , Ilona Kryczek 1 , Shaomeng Wang 12 , Lieping Chen 13 , Weiping Zou 14
Affiliation
|
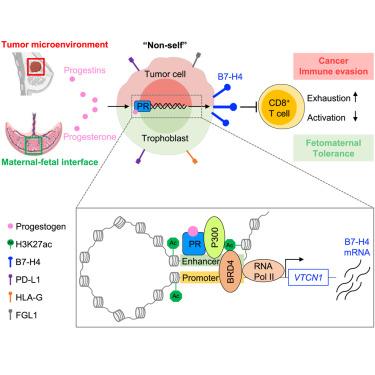
Immune tolerance mechanisms are shared in cancer and pregnancy. Through cross-analyzing single-cell RNA-sequencing data from multiple human cancer types and the maternal-fetal interface, we found B7-H4 () is an onco-fetal immune tolerance checkpoint. We showed that genetic deficiency of B7-H4 resulted in immune activation and fetal resorption in allogeneic pregnancy models. Analogously, B7-H4 contributed to MPA/DMBA-induced breast cancer progression, accompanied by CD8 T cell exhaustion. Female hormone screening revealed that progesterone stimulated B7-H4 expression in placental and breast cancer cells. Mechanistically, progesterone receptor (PR) bound to a newly identified −58 kb enhancer, thereby mediating B7-H4 transcription via the PR-P300-BRD4 axis. PR antagonist or BRD4 degrader potentiated immunotherapy in a murine B7-H4 breast cancer model. Thus, our work unravels a mechanistic and biological connection of a female sex hormone (progesterone) to onco-fetal immune tolerance via B7-H4 and suggests that the PR-P300-BRD4 axis is targetable for treating B7-H4 cancer.
中文翻译:

孕激素驱动的 B7-H4 有助于肿瘤胎儿免疫耐受
免疫耐受机制在癌症和妊娠中是共有的。通过交叉分析多种人类癌症类型和母胎界面的单细胞 RNA 测序数据,我们发现 B7-H4 () 是一个肿瘤-胎儿免疫耐受检查点。我们发现,B7-H4 的遗传缺陷会导致同种异体妊娠模型中的免疫激活和胎儿吸收。类似地,B7-H4 促进 MPA/DMBA 诱导的乳腺癌进展,并伴有 CD8 T 细胞耗竭。女性激素筛查显示,黄体酮可刺激胎盘癌细胞和乳腺癌细胞中 B7-H4 的表达。从机制上讲,孕酮受体 (PR) 与新鉴定的 -58 kb 增强子结合,从而通过 PR-P300-BRD4 轴介导 B7-H4 转录。 PR 拮抗剂或 BRD4 降解剂在小鼠 B7-H4 乳腺癌模型中增强免疫治疗。因此,我们的工作揭示了女性性激素(黄体酮)通过 B7-H4 与肿瘤胎儿免疫耐受之间的机制和生物学联系,并表明 PR-P300-BRD4 轴可作为治疗 B7-H4 癌症的目标。
更新日期:2024-07-04
中文翻译:

孕激素驱动的 B7-H4 有助于肿瘤胎儿免疫耐受
免疫耐受机制在癌症和妊娠中是共有的。通过交叉分析多种人类癌症类型和母胎界面的单细胞 RNA 测序数据,我们发现 B7-H4 () 是一个肿瘤-胎儿免疫耐受检查点。我们发现,B7-H4 的遗传缺陷会导致同种异体妊娠模型中的免疫激活和胎儿吸收。类似地,B7-H4 促进 MPA/DMBA 诱导的乳腺癌进展,并伴有 CD8 T 细胞耗竭。女性激素筛查显示,黄体酮可刺激胎盘癌细胞和乳腺癌细胞中 B7-H4 的表达。从机制上讲,孕酮受体 (PR) 与新鉴定的 -58 kb 增强子结合,从而通过 PR-P300-BRD4 轴介导 B7-H4 转录。 PR 拮抗剂或 BRD4 降解剂在小鼠 B7-H4 乳腺癌模型中增强免疫治疗。因此,我们的工作揭示了女性性激素(黄体酮)通过 B7-H4 与肿瘤胎儿免疫耐受之间的机制和生物学联系,并表明 PR-P300-BRD4 轴可作为治疗 B7-H4 癌症的目标。

































 京公网安备 11010802027423号
京公网安备 11010802027423号