Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-07-03 , DOI: 10.1002/adsc.202400738 Anushka Rastogi 1 , Mohit Kumar 1 , Manoj Kumar Gangwar 2 , Dipankar Koley 3
|
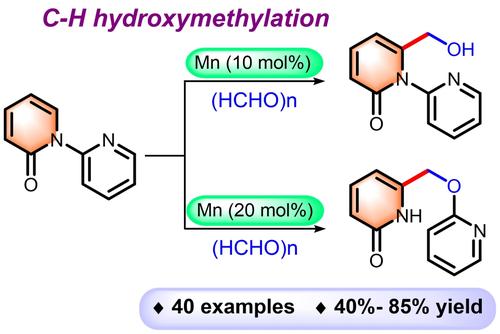
Pyridine-directed regioselective C−H hydroxymethylation to pyridones and isoquinolones using para-formaldehyde has been developed under manganese catalysis, offering a wide variety of hydroxymethylated products in 40–85% yields. This operationally simple methodology proceeds in one step using an earth- abundant first-row transition metal-catalyst without the generation of unwanted salt wastes. A mechanistic study revealed that the C−H metalation step is reversible but not the rate-determining step. The introduction of hydroxymethyl group in the biologically relevant scaffolds could be used as an intermediate for valuable synthetic transformations.
中文翻译:

锰通过 C−H 活化催化位点选择性羟甲基化生成 2-吡啶酮和异喹诺酮
在锰催化下,已开发出使用多聚甲醛进行吡啶定向区域选择性 C−H 羟甲基化生成吡啶酮和异喹诺酮的方法,可提供多种羟甲基化产物,产率为 40-85%。这种操作简单的方法使用地球上丰富的第一行过渡金属催化剂一步进行,不会产生不需要的盐废物。机理研究表明,C−H 金属化步骤是可逆的,但不是速率决定步骤。在生物学相关的支架中引入羟甲基可以用作有价值的合成转化的中间体。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号