当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric difluoroalkylation via Michael addition of an in situ generated difluoroenol intermediate
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-03 , DOI: 10.1039/d4qo00987h Xiongda Xie 1 , Gang Chen 1 , Jingjing Huang 2 , Yongqiang Liu 3 , Xinfang Xu 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-07-03 , DOI: 10.1039/d4qo00987h Xiongda Xie 1 , Gang Chen 1 , Jingjing Huang 2 , Yongqiang Liu 3 , Xinfang Xu 1
Affiliation
|
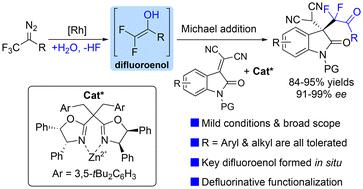
A dirhodium and chiral Zn-complex co-catalyzed asymmetric difluoroalkylation of isatylidene malononitriles via a Michael-type interception of α,α-difluoroenol species, which are generated in situ from trifluoromethyl diazo compounds and water in the presence of Rh2(esp)2, has been disclosed. This reaction provides an efficient approach for the synthesis of fluorinated oxindoles containing a chiral quaternary carbon center in generally good yields and with excellent stereoselectivities (91%–99% ee). Compared with difluoroenoxysilane, this difluoroenol intermediate showed higher reactivity and enantioselectivity under mild conditions.
中文翻译:

通过原位生成的二氟烯醇中间体的迈克尔加成进行不对称二氟烷基化
二铑和手性锌络合物通过 α,α-二氟烯醇物质的 Michael 型截获共催化异亚莠基丙二腈的不对称二氟烷基化,该物质是在 Rh 存在下由三氟甲基重氮化合物和水原位生成的 2 ,已公开。该反应为合成含有手性季碳中心的氟化羟吲哚提供了一种有效的方法,通常产率良好,并且具有优异的立体选择性(91%–99% ee)。与二氟烯氧基硅烷相比,该二氟烯醇中间体在温和条件下表现出更高的反应活性和对映选择性。
更新日期:2024-07-04
中文翻译:

通过原位生成的二氟烯醇中间体的迈克尔加成进行不对称二氟烷基化
二铑和手性锌络合物通过 α,α-二氟烯醇物质的 Michael 型截获共催化异亚莠基丙二腈的不对称二氟烷基化,该物质是在 Rh 存在下由三氟甲基重氮化合物和水原位生成的 2 ,已公开。该反应为合成含有手性季碳中心的氟化羟吲哚提供了一种有效的方法,通常产率良好,并且具有优异的立体选择性(91%–99% ee)。与二氟烯氧基硅烷相比,该二氟烯醇中间体在温和条件下表现出更高的反应活性和对映选择性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号