Journal of Fluorescence ( IF 2.6 ) Pub Date : 2024-07-03 , DOI: 10.1007/s10895-024-03829-z Deepa H Krishne 1 , Kalpana Sharma 1 , A Jagannatha Reddy 1 , V V Koppal 2
|
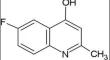
This study investigates the interaction between titanium oxide nanoparticles (TiO2 NPs) and the heterocyclic fluorophore 6-fluoro,4-hydroxy,2-methylquinoline (6-FHMQ), aiming to understand fluorescence quenching mechanisms and thermodynamic characteristics. Spectroscopic techniques including spectrofluorometry (FL) and spectrophotometry (UV–Vis) were used, with a lifetime decay (τ) of 0.18 ns for 6-FHMQ measured using time correlated single photon counting (TCSPC). The interaction between 6-FHMQ and TiO2 NPs revealed a mix of static and dynamic fluorescence quenching mechanisms, with increasing quenching constants (Ksv) and a higher bimolecular quenching rate constant (Kq). The dynamic nature was highlighted by a temperature-dependent increase in binding sites from 1 to ~ 2. Spontaneous complexation was affirmed by negative change in free energy (ΔG), with negative change in enthalpy (ΔH) and a positive change in entropy (ΔS) values indicating favorable electrostatic and ionic interactions. The impact of varying TiO2 NP concentrations on 6-FHMQ absorption was analyzed using the Benesi-Hildbrand equation, with a quantum yield of 0.61 determined. By forster resonance energy transfer (FRET) theory, the proximity between 6-FHMQ and TiO2 NPs was found to be less than 70 Å. Ground and excited state dipole moments of 6-FHMQ in different solvents were calculated to demonstrate solvent sensing ability and charge transfer properties. Ultimately, this study serves as a testament to the power of scientific innovation in the realms of drug delivery and tissue engineering.
中文翻译:

探索 6-氟、4-羟基、2-甲基喹啉与 TiO2 纳米粒子的分子结合机制:光谱、热力学和溶剂化变色效应的见解
本研究研究了二氧化钛纳米粒子(TiO 2 NPs)与杂环荧光团6-氟,4-羟基,2-甲基喹啉(6-FHMQ)之间的相互作用,旨在了解荧光猝灭机制和热力学特性。使用包括荧光分光光度法 (FL) 和分光光度法 (UV-Vis) 在内的光谱技术,使用时间相关单光子计数 (TCSPC) 测量 6-FHMQ 的寿命衰减 (τ) 为 0.18 ns。 6-FHMQ 和 TiO 2 NP 之间的相互作用揭示了静态和动态荧光猝灭机制的混合,具有增加的猝灭常数 (Ksv) 和更高的双分子猝灭速率常数 (Kq)。结合位点从 1 到 ~ 2 的温度依赖性增加突出了动态性质。自由能 (ΔG) 的负变化、焓 (ΔH) 的负变化和熵 (ΔS) 的正变化证实了自发络合。 ) 值表明有利的静电和离子相互作用。使用Benesi-Hildbrand方程分析不同TiO 2 NP浓度对6-FHMQ吸收的影响,确定量子产率为0.61。通过福斯特共振能量转移(FRET)理论,发现6-FHMQ和TiO 2 NP之间的接近度小于70 Å。计算了 6-FHMQ 在不同溶剂中的基态和激发态偶极矩,以证明溶剂传感能力和电荷转移特性。最终,这项研究证明了药物输送和组织工程领域科学创新的力量。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号