Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Synthesis of 2-Benzylideneindolin-3-ones via a Nucleophilic/Rearrangement/Azide–Alkene Cascade Reaction
Synlett ( IF 1.7 ) Pub Date : 2024-07-02 , DOI: 10.1055/a-2341-9185 Quan Tang 1 , Hua Wang 2 , Tianyu Long 3 , Han-Han Kong 1 , Qing-Qing Yang 4 , Nianyu Huang 5 , Long Wang 6
中文翻译:

通过亲核/重排/叠氮化物-烯烃级联反应无金属合成 2-亚苄基吲哚啉-3-酮
更新日期:2024-07-03
Synlett ( IF 1.7 ) Pub Date : 2024-07-02 , DOI: 10.1055/a-2341-9185 Quan Tang 1 , Hua Wang 2 , Tianyu Long 3 , Han-Han Kong 1 , Qing-Qing Yang 4 , Nianyu Huang 5 , Long Wang 6
Affiliation
|
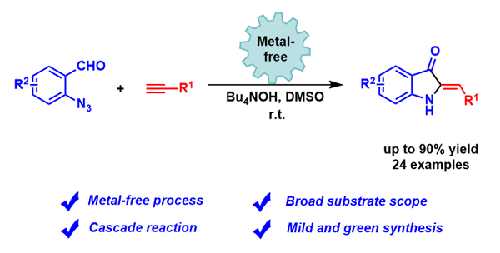
A metal-free synthesis of 2-benzylideneindolin-3-ones through a formal [4+1] annulation from o-azidobenzaldehydes and terminal alkynes has been developed. The method features operational simplicity, mild reaction conditions, and ready availability of starting materials. A broad range of 2-benzylideneindolin-3-ones were prepared in moderate to excellent yields. Mechanistic studies indicated that the reaction might proceed through a nucleophilic addition/rearrangement/azide–alkene cycloaddition pathway to produce 2-benzylideneindolin-3-ones.
中文翻译:

通过亲核/重排/叠氮化物-烯烃级联反应无金属合成 2-亚苄基吲哚啉-3-酮
已经开发出通过邻叠氮基苯甲醛和末端炔烃的正式[4+1]环化来无金属合成2-亚苄基吲哚啉-3-酮。该方法具有操作简单、反应条件温和、起始原料易得等特点。以中等至优异的产率制备了多种 2-亚苄基二氢吲哚-3-酮。机理研究表明该反应可能通过亲核加成/重排/叠氮-烯烃环加成途径进行,生成2-亚苄基吲哚啉-3-酮。













































 京公网安备 11010802027423号
京公网安备 11010802027423号