当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Universality of critical active site glutamate as an acid–base catalyst in serine hydroxymethyltransferase function
Chemical Science ( IF 7.6 ) Pub Date : 2024-07-03 , DOI: 10.1039/d4sc03187c Victoria N. Drago 1 , Robert S. Phillips 2, 3 , Andrey Kovalevsky 1
Chemical Science ( IF 7.6 ) Pub Date : 2024-07-03 , DOI: 10.1039/d4sc03187c Victoria N. Drago 1 , Robert S. Phillips 2, 3 , Andrey Kovalevsky 1
Affiliation
|
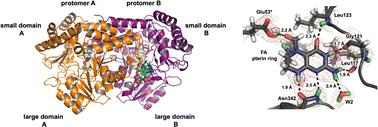
Serine hydroxymethyltransferase (SHMT) is a key enzyme in the one-carbon metabolic pathway, utilizing the vitamin B6 derivative pyridoxal 5′-phosphate (PLP) and vitamin B9 derivative tetrahydrofolate (THF) coenzymes to produce essential biomolecules. Many types of cancer utilize SHMT in metabolic reprogramming, exposing the enzyme as a compelling target for antimetabolite chemotherapies. In pursuit of elucidating the catalytic mechanism of SHMT to aid in the design of SHMT-specific inhibitors, we have used room-temperature neutron crystallography to directly determine the protonation states in a model enzyme Thermus thermophilus SHMT (TthSHMT), which exhibits a conserved active site compared to human mitochondrial SHMT2 (hSHMT2). Here we report the analysis of TthSHMT, with PLP in the internal aldimine form and bound THF-analog, folinic acid (FA), by neutron crystallography to reveal H atom positions in the active site, including PLP and FA. We observed protonated catalytic Glu53 revealing its ability to change protonation state upon FA binding. Furthermore, we obtained X-ray structures of TthSHMT-Gly/FA, TthSHMT-L-Ser/FA, and hSHMT2-Gly/FA ternary complexes with the PLP-Gly or PLP-L-Ser external aldimines to analyze the active site configuration upon PLP reaction with an amino acid substrate and FA binding. Accurate mapping of the active site protonation states together with the structural information gained from the ternary complexes allow us to suggest an essential role of the gating loop conformational changes in the SHMT function and to propose Glu53 as the universal acid-base catalyst in both THF-independent and THF-dependent activities of SHMT.
中文翻译:

关键活性位点谷氨酸作为丝氨酸羟甲基转移酶功能酸碱催化剂的普遍性
丝氨酸羟甲基转移酶 (SHMT) 是一碳代谢途径中的关键酶,利用维生素 B 6 衍生物吡哆醛 5'-磷酸 (PLP) 和维生素 B 9 衍生物四氢叶酸 ( THF)辅酶来产生必需的生物分子。许多类型的癌症在代谢重编程中利用 SHMT,使该酶成为抗代谢化疗的引人注目的靶标。为了阐明 SHMT 的催化机制以帮助设计 SHMT 特异性抑制剂,我们使用室温中子晶体学直接测定模型酶嗜热栖热菌 SHMT (TthSHMT) 中的质子化状态,该酶表现出保守的活性与人类线粒体 SHMT2 (hSHMT2) 相比的位点。在这里,我们报告了 TthSHMT 的分析,其中 PLP 为内部醛亚胺形式,并结合了 THF 类似物亚叶酸 (FA),通过中子晶体学揭示了活性位点中的 H 原子位置,包括 PLP 和 FA。我们观察到质子化的催化 Glu53 揭示了其在 FA 结合后改变质子化状态的能力。此外,我们还获得了 TthSHMT-Gly/FA、TthSHMT-L-Ser/FA 和 hSHMT2-Gly/FA 三元复合物与 PLP-Gly 或 PLP-L-Ser 外部醛亚胺的 X 射线结构,以分析活性位点构型PLP 与氨基酸底物反应并结合 FA。活性位点质子化状态的准确映射以及从三元配合物获得的结构信息使我们能够提出门环构象变化在 SHMT 功能中的重要作用,并提出 Glu53 作为 THF- 中的通用酸碱催化剂。 SHMT 的独立活动和 THF 依赖性活动。
更新日期:2024-07-03
中文翻译:

关键活性位点谷氨酸作为丝氨酸羟甲基转移酶功能酸碱催化剂的普遍性
丝氨酸羟甲基转移酶 (SHMT) 是一碳代谢途径中的关键酶,利用维生素 B 6 衍生物吡哆醛 5'-磷酸 (PLP) 和维生素 B 9 衍生物四氢叶酸 ( THF)辅酶来产生必需的生物分子。许多类型的癌症在代谢重编程中利用 SHMT,使该酶成为抗代谢化疗的引人注目的靶标。为了阐明 SHMT 的催化机制以帮助设计 SHMT 特异性抑制剂,我们使用室温中子晶体学直接测定模型酶嗜热栖热菌 SHMT (TthSHMT) 中的质子化状态,该酶表现出保守的活性与人类线粒体 SHMT2 (hSHMT2) 相比的位点。在这里,我们报告了 TthSHMT 的分析,其中 PLP 为内部醛亚胺形式,并结合了 THF 类似物亚叶酸 (FA),通过中子晶体学揭示了活性位点中的 H 原子位置,包括 PLP 和 FA。我们观察到质子化的催化 Glu53 揭示了其在 FA 结合后改变质子化状态的能力。此外,我们还获得了 TthSHMT-Gly/FA、TthSHMT-L-Ser/FA 和 hSHMT2-Gly/FA 三元复合物与 PLP-Gly 或 PLP-L-Ser 外部醛亚胺的 X 射线结构,以分析活性位点构型PLP 与氨基酸底物反应并结合 FA。活性位点质子化状态的准确映射以及从三元配合物获得的结构信息使我们能够提出门环构象变化在 SHMT 功能中的重要作用,并提出 Glu53 作为 THF- 中的通用酸碱催化剂。 SHMT 的独立活动和 THF 依赖性活动。











































 京公网安备 11010802027423号
京公网安备 11010802027423号