当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Negative cooperativity in the formation of H-bond networks involving primary anilines
Chemical Science ( IF 7.6 ) Pub Date : 2024-07-03 , DOI: 10.1039/d4sc03719g Fergal E. Hanna 1 , Alexander J. Root 1 , Markus Schade 2 , Christopher A. Hunter 1
Chemical Science ( IF 7.6 ) Pub Date : 2024-07-03 , DOI: 10.1039/d4sc03719g Fergal E. Hanna 1 , Alexander J. Root 1 , Markus Schade 2 , Christopher A. Hunter 1
Affiliation
|
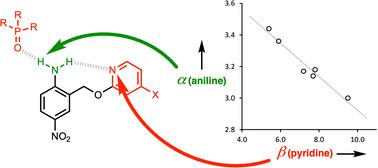
Networks of H-bonds can show non-additive behaviour, where the strength of one interaction perturbs another. The magnitude of such cooperative effects can be quantified by measuring the effect of the presence of an intramolecular H-bond at one site on a molecule on the association constant for formation of an intermolecular H-bond at another site. This approach has been used to quantify the cooperativity associated with the interaction of a primary amine with two H-bond acceptors. A series of compounds that have an intramolecular H-bond between an aniline NH2 group and a pyridine nitrogen were prepared, using polarising substituents on the pyridine ring to vary the strength of the intramolecular H-bond. The presence of the intramolecular interaction was confirmed by X-ray crystallography in the solid state and NMR spectroscopy in n-octane solution. UV-vis absorption titrations were used to measure the association constants for formation of an intermolecular H-bond with tri-n-butyl phosphine oxide in n-octane. Electron-donating substituents on the pyridine ring, which increase the strength of the intramolecular H-bond, were found to decrease the strength of the intermolecular H-bond between the aniline and the phosphine oxide. The results were used to determine the H-bond donor parameters for the anilines, α, and there is a linear relationship between the values of α and the H-bond acceptor parameter of the pyridine group involved in the intramolecular H-bond, β. The slope of this relationship was used to determine the cooperativity parameter (κ = −0.10), which quantifies the negative allosteric cooperativity between the two H-bonding interactions. Calculated molecular electrostatic potential surfaces of the anilines quantitatively reproduce the experimental result, which suggests that effects are electrostatic in origin, either due to polarisation of the NH bonds or due to secondary electrostatic interactions between the two H-bond acceptors.
中文翻译:

涉及伯苯胺的氢键网络形成中的负协同作用
氢键网络可以表现出非加和行为,其中一种相互作用的强度会扰乱另一种相互作用。这种协同效应的大小可以通过测量分子上一个位点处分子内氢键的存在对另一位点处分子间氢键形成的缔合常数的影响来量化。该方法已用于量化与伯胺与两个氢键受体相互作用相关的协同性。利用吡啶环上的极化取代基来改变分子内氢键的强度,制备了一系列在苯胺NH 2 基团和吡啶氮之间具有分子内氢键的化合物。通过固态X射线晶体学和正辛烷溶液中的NMR光谱证实了分子内相互作用的存在。使用紫外可见吸收滴定法测量正辛烷中与三正丁基氧化膦形成分子间氢键的缔合常数。吡啶环上的给电子取代基可增加分子内氢键的强度,但会降低苯胺和氧化膦之间的分子间氢键的强度。结果用于确定苯胺的氢键供体参数α,并且α值与参与分子内氢键的吡啶基团的氢键受体参数β之间存在线性关系。这种关系的斜率用于确定协同参数 (κ = -0.10),该参数量化两个氢键相互作用之间的负变构协同性。 计算得出的苯胺分子静电势表面定量再现了实验结果,这表明效应本质上是静电效应,要么是由于 NH 键的极化,要么是由于两个氢键受体之间的二次静电相互作用。
更新日期:2024-07-03
中文翻译:

涉及伯苯胺的氢键网络形成中的负协同作用
氢键网络可以表现出非加和行为,其中一种相互作用的强度会扰乱另一种相互作用。这种协同效应的大小可以通过测量分子上一个位点处分子内氢键的存在对另一位点处分子间氢键形成的缔合常数的影响来量化。该方法已用于量化与伯胺与两个氢键受体相互作用相关的协同性。利用吡啶环上的极化取代基来改变分子内氢键的强度,制备了一系列在苯胺NH 2 基团和吡啶氮之间具有分子内氢键的化合物。通过固态X射线晶体学和正辛烷溶液中的NMR光谱证实了分子内相互作用的存在。使用紫外可见吸收滴定法测量正辛烷中与三正丁基氧化膦形成分子间氢键的缔合常数。吡啶环上的给电子取代基可增加分子内氢键的强度,但会降低苯胺和氧化膦之间的分子间氢键的强度。结果用于确定苯胺的氢键供体参数α,并且α值与参与分子内氢键的吡啶基团的氢键受体参数β之间存在线性关系。这种关系的斜率用于确定协同参数 (κ = -0.10),该参数量化两个氢键相互作用之间的负变构协同性。 计算得出的苯胺分子静电势表面定量再现了实验结果,这表明效应本质上是静电效应,要么是由于 NH 键的极化,要么是由于两个氢键受体之间的二次静电相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号