当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aromaticity transfer in an annulated 1,4,2-diazaborole: facile access to Cs symmetric 1,4,2,5-diazadiborinines
Chemical Communications ( IF 4.3 ) Pub Date : 2024-07-02 , DOI: 10.1039/d4cc02414a Vignesh Pattathil 1 , Conor Pranckevicius 1
Chemical Communications ( IF 4.3 ) Pub Date : 2024-07-02 , DOI: 10.1039/d4cc02414a Vignesh Pattathil 1 , Conor Pranckevicius 1
Affiliation
|
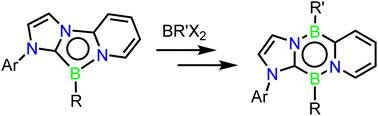
A tricyclic annulated 1,4,2-diazaborole is readily accessed via reaction of a bidentate pyridyl-carbene ligand with MesBBr2 followed by reduction. Dearomatization of the flanking rings is shown to increase reactivity of this heterocycle in the form of a B-centred alkylation with MeI. Its reaction with hydrido-, fluoro-, and chloro-boranes reveal an unprecedented ring expansion reaction to form a diverse family of B2C2N2 heterocycles, reduction of which allows facile access to the first examples of Cs symmetric 1,4,2,5-diazadiborinines. DFT calculations have shed light on the electronic structures of the reduced species and provide insight into mechanistic aspects of the observed ring-expansion.
中文翻译:

环状 1,4,2-二氮杂硼杂环戊烯中的芳香性转移:轻松获得 Cs 对称 1,4,2,5-二氮杂二硼啉
通过双齿吡啶基卡宾配体与 MesBBr 2 反应,然后还原,可以轻松获得三环环状 1,4,2-二氮硼杂环。侧翼环的脱芳构化以与 MeI 进行的以 B 为中心的烷基化形式增加了该杂环的反应性。它与氢硼烷、氟硼烷和氯硼烷的反应揭示了前所未有的扩环反应,形成了多种 B 2 C 2 N 2 杂环家族,对其进行还原可以轻松获得 C s 对称 1,4,2,5-二氮杂二硼啉的第一个例子。 DFT 计算揭示了还原物质的电子结构,并提供了对所观察到的环膨胀的机制方面的深入了解。
更新日期:2024-07-02
中文翻译:

环状 1,4,2-二氮杂硼杂环戊烯中的芳香性转移:轻松获得 Cs 对称 1,4,2,5-二氮杂二硼啉
通过双齿吡啶基卡宾配体与 MesBBr 2 反应,然后还原,可以轻松获得三环环状 1,4,2-二氮硼杂环。侧翼环的脱芳构化以与 MeI 进行的以 B 为中心的烷基化形式增加了该杂环的反应性。它与氢硼烷、氟硼烷和氯硼烷的反应揭示了前所未有的扩环反应,形成了多种 B 2 C 2 N 2 杂环家族,对其进行还原可以轻松获得 C s 对称 1,4,2,5-二氮杂二硼啉的第一个例子。 DFT 计算揭示了还原物质的电子结构,并提供了对所观察到的环膨胀的机制方面的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号