Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Major-groove sequence-specific RNA recognition by LoaP, a paralog of transcription elongation factor NusG
Structure ( IF 4.4 ) Pub Date : 2024-07-02 , DOI: 10.1016/j.str.2024.06.001 Amr Elghondakly 1 , Madison D Jermain 2 , Wade C Winkler 3 , Adrian R Ferré-D'Amaré 1
Structure ( IF 4.4 ) Pub Date : 2024-07-02 , DOI: 10.1016/j.str.2024.06.001 Amr Elghondakly 1 , Madison D Jermain 2 , Wade C Winkler 3 , Adrian R Ferré-D'Amaré 1
Affiliation
|
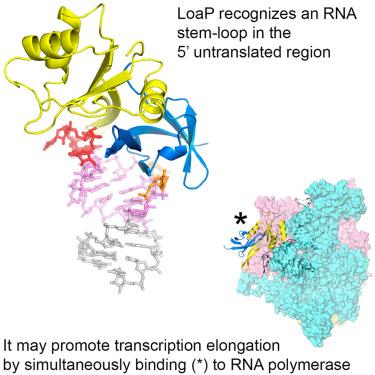
LoaP is a member of the universal NusG protein family. Previously, we reported that unlike other characterized homologs, LoaP binds RNA sequence-specifically, recognizing a stem-loop in the 5′-untranslated region of operons it regulates. To elucidate how this NusG homolog acquired this ability, we now determined the co-crystal structure of Thermoanaerobacter pseudethanolicus LoaP bound to its cognate 26-nucleotide dfn RNA element. Our structure reveals that the LoaP C-terminal KOW domain recognizes the helical portion of the RNA by docking into a broadened major groove, while a protruding β-hairpin of the N-terminal NusG-like domain binds the UNCG tetraloop capping the stem-loop. Major-groove RNA recognition is unusual and is made possible by conserved features of the dfn hairpin. Superposition with structures of other NusG proteins implies that LoaP can bind concurrently to the dfn RNA and the transcription elongation complex, suggesting a new level of co-transcriptional regulation by proteins of this conserved family.
中文翻译:

LoaP(转录延伸因子 NusG 的旁系同源物)识别主沟序列特异性 RNA
LoaP 是通用 NusG 蛋白家族的成员。之前,我们报道过,与其他特征同源物不同,LoaP 特异性结合 RNA 序列,识别其调节的操纵子 5'-非翻译区的茎环。为了阐明该 NusG 同源物如何获得这种能力,我们现在确定了拟乙醇嗜热厌氧杆菌 LoaP 与其同源 26 核苷酸 dfn RNA 元件结合的共晶结构。我们的结构表明,LoaP C 端 KOW 结构域通过对接至加宽的主沟来识别 RNA 的螺旋部分,而 N 端 NusG 样结构域的突出 β 发夹结合覆盖茎环的 UNCG 四环。主沟 RNA 识别是不寻常的,它是通过 dfn 发夹的保守特征实现的。与其他 NusG 蛋白结构的叠加意味着 LoaP 可以同时结合 dfn RNA 和转录延伸复合物,表明该保守家族蛋白的共转录调节达到了新水平。
更新日期:2024-07-02
中文翻译:

LoaP(转录延伸因子 NusG 的旁系同源物)识别主沟序列特异性 RNA
LoaP 是通用 NusG 蛋白家族的成员。之前,我们报道过,与其他特征同源物不同,LoaP 特异性结合 RNA 序列,识别其调节的操纵子 5'-非翻译区的茎环。为了阐明该 NusG 同源物如何获得这种能力,我们现在确定了拟乙醇嗜热厌氧杆菌 LoaP 与其同源 26 核苷酸 dfn RNA 元件结合的共晶结构。我们的结构表明,LoaP C 端 KOW 结构域通过对接至加宽的主沟来识别 RNA 的螺旋部分,而 N 端 NusG 样结构域的突出 β 发夹结合覆盖茎环的 UNCG 四环。主沟 RNA 识别是不寻常的,它是通过 dfn 发夹的保守特征实现的。与其他 NusG 蛋白结构的叠加意味着 LoaP 可以同时结合 dfn RNA 和转录延伸复合物,表明该保守家族蛋白的共转录调节达到了新水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号