Journal of Ecology ( IF 5.3 ) Pub Date : 2024-07-02 , DOI: 10.1111/1365-2745.14362 Ester González de Andrés 1 , Antonio Gazol 1 , J. Julio Camarero 1 , José Antonio Bonet 2, 3 , Maria Caballol 2, 3 , Alexandra Ceausu 3 , Jonàs Oliva 2, 3
|
1 INTRODUCTION
Pathogens play a major role in forest dynamics because of their capacity to induce tree mortality (Gilbert, 2002; Oliva et al., 2020). Root rot pathogens are parasitic necrotrophic fungi that form long-lived individuals (referred to as genets), which can infect more than one tree at once (Garbelotto & Gonthier, 2013). Their large size allows them to access large energy pools and build up a sufficient inoculum potential required to kill the root system of large and vigorous trees (Linares et al., 2010; Oliva et al., 2020). The resulting mortality pockets or forest gaps are identified by the presence of dead and dying trees surrounded by an apparently healthy forest (Bendel, Kienast, Bugmann, et al., 2006). Some features render root-rot-induced gaps different to gaps created by other abiotic and biotic disturbances such as bark beetles or snow and windstorms, including slow rate of formation, small size and increased light reaching the forest ground due to the canopy openings resulting from frequent wind-thrown trees (Redfern & Stenlid, 1998).
Despite their conspicuous presence in coniferous forests, knowledge on how trees respond to gap formation related to root rot pathogens is largely incomplete. Root rots are host-specific and usually harbour decomposing abilities, so they can survive long after tree death, thus providing a competitive advantage to non-host tree species (Asiegbu et al., 2005; Oliva et al., 2020), and triggering regeneration of shade-tolerant species (Bendel, Kienast, Rigling, et al., 2006; Navarro-Cerrillo et al., 2014). In contrast, root rot infection often expands beyond the actual limit of the gap, thus preventing supressed and surrounding trees from displaying growth releases (Bendz-Hellgren & Stenlid, 1995; Cherubini et al., 2002). Therefore, root rots can both create gaps and prevent their closure from the side. Furthermore, the interaction between pathogen effects and abiotic factors such as drought stress can significantly increase tree mortality (Camarero et al., 2015; Gomez-Gallego et al., 2022; Oliva et al., 2014). However, the role played by the interaction between root rots and drought stress in the formation and maintenance of forest gaps is poorly understood. The putative links between mortality and drought are of particular concern for tree species located at the xeric limit of the species distribution (Burgess et al., 2022; Hernández et al., 2019).
Little is known about the effects of the gap below-ground and how soil biotic and abiotic components are affected (Nygaard et al., 2018). Reduced canopy cover as a consequence of defoliation and tree death could modify fungal composition and community structure (Tomao et al., 2020). Previous studies have reported reductions in ectomycorrhizal biomass in connection with worsening health conditions in the canopy (Castaño et al., 2020; Wallander et al., 2010). Besides, studies involving interactions between bark beetle-induced tree dieback and soil microbial communities reported increases in saprophytic taxa and the reduction in symbiotic fungi (Štursová et al., 2014). However, nothing is known relating root rots and whether some mycorrhiza could benefit or suffer from such openings (Chen et al., 2022). The impact on mycorrhiza can also depend on the position of the trees within the gap. For instance, living but presumably infected trees at the edge of the gap may also have problems sustaining mycorrhiza (Anthony et al., 2022). These below-ground processes can cause feedback effects on the gap formation, as the loss of mycorrhiza may further increase the susceptibility of trees to other biotic and abiotic stressors (Baldrian, 2017; Velmala et al., 2018).
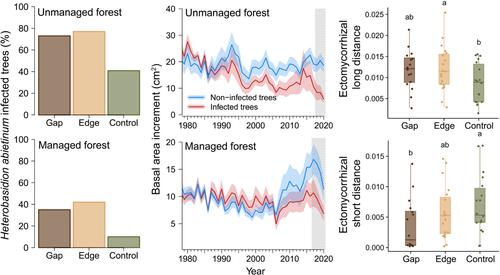
One of the most common root rot pathogens associated with mortality gaps in conifer forests is the genera Heterobasidion Bref. (Bendel, Kienast, Bugmann, et al., 2006; Garbelotto, 2004), which is the most economically loss-making species in the Northern Hemisphere (Garbelotto & Gonthier, 2013). It is very prevalent in managed stands, as it easily colonizes stumps created after thinning and clear-cuts, providing new available resources (Oliva et al., 2009). Hence, the role of historical management needs to be considered when studying the dynamics of Heterobasidion created gaps (Oliva et al., 2010). In this study, we explored the above- (trees) and below-ground (soil microbiome) dynamics of forest gaps caused by Heterobasidion abietinum Niemelä & Korhonen in silver fir (Abies alba Mill.) forests in the Spanish Pyrenees. Although silver fir is regarded as a relatively tolerant species to root rot (Greig et al., 2001), H. abietinum is widespread in these forests, being present in more than 30% of dead trees (Oliva & Colinas, 2007). On top of this significant pathogen pressure, fir forests in the Pyrenees have been struck by major drought spells since the 1980s together with widespread symptoms of decline (Burgess et al., 2022; Camarero et al., 2015; Oliva & Colinas, 2007). Therefore, Pyrenean fir forests constitute a good setting to analyse the interaction between the rot root pathogen and drought on trees and soil communities.
This research aimed (1) to evaluate mortality patterns in response to gap formation; (2) to assess drought impacts on tree growth considering their H. abietinum infection and management history; and (3) to characterize the impact of gap formation on soil microbiota and soil fertility. For that purpose, we combined dendrochronological techniques and metabarcoding of soil environmental DNA to study six gaps located in two forests with contrasting management situations in the Spanish Pyrenees. We hypothesized that (1) root rot gaps are the result of two processes: the pathogens killing trees and opening the canopy, and the pathogen infecting trees at the edge and preventing canopy closure; (2) drought influences gap establishment and further expansion as infected trees will be more affected and less likely to recover from periods with water stress than uninfected trees; and (3) gap formation changes soil biotic and abiotic conditions.
中文翻译:

根腐病病原体引起的间隙促进了森林和土壤真菌群落动态
1 简介
病原体在森林动态中发挥着重要作用,因为它们能够导致树木死亡(Gilbert, 2002 ;Oliva 等, 2020 )。根腐病病原体是寄生性坏死营养真菌,可形成长寿个体(称为基因),可以同时感染一棵以上的树(Garbelotto & Gonthier, 2013 )。它们的大尺寸使它们能够获得巨大的能量库,并建立足够的接种潜力,以杀死高大而有活力的树木的根系(Linares等人, 2010年;Oliva等人, 2020年)。由此产生的死亡区域或森林间隙是通过周围明显健康的森林周围的死亡和垂死的树木来确定的(Bendel、Kienast、Bugmann 等人, 2006 年)。一些特征使得根腐引起的间隙不同于其他非生物和生物干扰(例如树皮甲虫或雪和风暴)造成的间隙,包括形成速度慢、尺寸小以及由于树冠开口而导致到达森林地面的光线增加。经常被风吹倒的树木(Redfern & Stenlid, 1998 )。
尽管它们在针叶林中的存在很明显,但关于树木如何应对与根腐病病原体相关的间隙形成的知识在很大程度上是不完整的。根腐病是宿主特异性的,通常具有分解能力,因此它们可以在树木死亡后长期存活,从而为非宿主树种提供竞争优势(Asiegbu et al., 2005 ;Oliva et al., 2020 ),并引发耐荫物种的再生(Bendel、Kienast、Rigling 等人, 2006 年;Navarro-Cerrillo 等人, 2014 年)。相反,根腐病感染通常会扩大到超出间隙的实际限制,从而阻止受抑制的树木和周围的树木表现出生长释放(Bendz-Hellgren&Stenlid, 1995 ;Cherubini等人, 2002 )。因此,根部腐烂既会产生间隙,又会阻止间隙从侧面闭合。此外,病原体效应与干旱胁迫等非生物因素之间的相互作用会显着增加树木死亡率(Camarero等, 2015 ;Gomez-Gallego等, 2022 ;Oliva等, 2014 )。然而,人们对根腐病和干旱胁迫之间的相互作用在林隙形成和维持中所起的作用知之甚少。对于位于物种分布干旱极限的树种,死亡率和干旱之间的假定联系尤其值得关注(Burgess 等人, 2022 年;Hernández 等人, 2019 年)。
关于地下间隙的影响以及土壤生物和非生物成分如何受到影响,人们知之甚少(Nygaard 等, 2018 )。落叶和树木死亡导致的树冠覆盖减少可能会改变真菌组成和群落结构(Tomao 等人, 2020 )。先前的研究报告称,外生菌根生物量的减少与冠层健康状况恶化有关(Castaño 等人, 2020 年;Wallander 等人, 2010 年)。此外,涉及树皮甲虫诱导的树木顶枯病和土壤微生物群落之间相互作用的研究报告称,腐生类群增加,共生真菌减少(Štursová et al., 2014 )。然而,目前尚不清楚根腐病以及某些菌根是否会因这种开口而受益或受损(Chen 等人, 2022 )。对菌根的影响还取决于树木在间隙内的位置。例如,间隙边缘的活树但可能被感染的树木也可能在维持菌根方面存在问题(Anthony et al., 2022 )。这些地下过程会对间隙形成产生反馈效应,因为菌根的丧失可能会进一步增加树木对其他生物和非生物应激源的敏感性(Baldrian, 2017 ;Velmala 等, 2018 )。
与针叶林死亡率差距相关的最常见根腐病病原体之一是异担子属Bref。 (Bendel, Kienast, Bugmann, et al., 2006 ; Garbelotto, 2004 ),是北半球经济损失最严重的物种(Garbelotto & Gonthier, 2013 )。它在管理林中非常普遍,因为它很容易在间伐和砍伐后形成的树桩上定居,提供新的可用资源(Oliva 等, 2009 )。因此,在研究异担子产生间隙的动态时,需要考虑历史管理的作用(Oliva et al., 2010 )。在这项研究中,我们探索了西班牙比利牛斯山脉银杉 ( Abies alba Mill.) 森林中由Heterobasidion abietinum Niemelä & Korhonen 引起的森林间隙的地上(树木)和地下(土壤微生物组)动态。尽管银杉被认为是对根腐病相对耐受的物种(Greig等, 2001 ),但H. abietinum在这些森林中广泛存在,存在于超过30%的死树中(Oliva&Colinas, 2007 )。除了这种巨大的病原体压力之外,比利牛斯山脉的冷杉林自 20 世纪 80 年代以来还遭受了严重干旱以及广泛的衰退症状(Burgess 等人, 2022 年;Camarero 等人, 2015 年;Oliva 和 Colinas, 2007 年) 。因此,比利牛斯冷杉森林构成了分析腐根病原体与树木和土壤群落干旱之间相互作用的良好环境。
这项研究的目的是 (1) 评估因差距形成而导致的死亡率模式; (2) 考虑松柏树感染和管理历史,评估干旱对树木生长的影响; (3) 表征间隙形成对土壤微生物群和土壤肥力的影响。为此,我们结合了树木年代学技术和土壤环境 DNA 元条形码技术,研究了位于西班牙比利牛斯山脉两座森林中的六个间隙,并对比了管理情况。我们假设(1)根腐间隙是两个过程的结果:病原体杀死树木并打开树冠,以及病原体感染边缘的树木并阻止树冠关闭; (2) 干旱会影响间隙的建立和进一步扩大,因为与未受感染的树木相比,受感染的树木受到的影响更大,而且从缺水时期恢复的可能性更小; (3)间隙的形成改变了土壤的生物和非生物条件。































 京公网安备 11010802027423号
京公网安备 11010802027423号